Dihydrocholesterol
Synonym(s):Dihydrocholesterol;β-Cholestanol;5α-Cholestan-3β-ol;3β-Hydroxy-5α-cholestane;5α-Cholestan-3?-ol
- CAS NO.:80-97-7
- Empirical Formula: C27H48O
- Molecular Weight: 388.68
- MDL number: MFCD00066413
- EINECS: 201-315-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
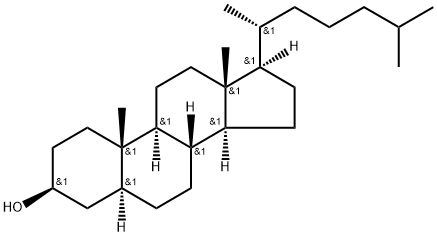
What is Dihydrocholesterol?
Description
Cholestanol is a cholesterol metabolite formed by oxidation and an intermediate in the biosynthesis of chenodeoxycholic acid . Cholestanol (10 μg/ml) induces apoptosis in cornea and lens epithelial cells and increases the activity of IL-1β converting enzyme (ICE) and CPP32 proteases. Dietary administration of 1% cholestanol to mice increases serum and liver cholestanol levels and leads to corneal opacities and gallstones and in rats it leads to cholestanol deposition in the cerebellum. Cholestanol levels are increased in plasma of patients with cerebrotendinous xanthomatosis (CTX), a disease characterized by a deficiency in the mitochondrial enzyme sterol 27-hydrolylase (CYP27A1) that leads to progressive neurological symptoms.
Chemical properties
white powder
The Uses of Dihydrocholesterol
5α-Cholestan-3β-ol is a carbon stanol formed from biohydrogenation of Cholesterol (C432501) in the gut. Studies have also examined the conversion of 5α-Cholestan-3β-ol catalyzed by 3-β-hydroxysteroid dehydrogenase of rat liver.
The Uses of Dihydrocholesterol
5alpha-Cholestan-3beta-ol is used as a standard in lipid analysis using HPLC. It acts as a derivitized steroid compound. It is used in agrochemical, pharmaceutical and dyestuff field .
What are the applications of Application
5α-Cholestan-3β-ol is a derivitized steroid compound
Definition
ChEBI: A cholestanoid that is (5alpha)-cholestane substituted by a beta-hydroxy group at position 3.
General Description
Cholestanol is a 5α-dihydro derivative of cholesterol. It acts as a marker for cholesterol absorption. Cholestanol is a steroid with 27 carbon atoms.
Biochem/physiol Actions
5α-Cholestan-3β-ol is derived from cholesterol by the action of intestinal microorganisms. It is known to induce the formation of gall stones in rabbits in the presence of sodium ions.
Purification Methods
Purify 5--cholestan-3-ol via acetylation, crystallisation and de-acetylation, then recrystallisation from EtOH or slightly aqueous EtOH, or MeOH. Its solubility is: 0.5% (MeOH) and 1% (EtOH) at 25o. [Mizutani & Whitten J Am Chem Soc 107 3621 1985.] The acetate has m 114-115o from EtOAc/MeOH and, [] D 20 +13o (c 2, CHCl3). [Bruce & Ralls Org Synth Col Vol II 191 1943, Beilstein 6 IV 3577.]
Properties of Dihydrocholesterol
| Melting point: | 138-142 °C |
| Boiling point: | 454.32°C (rough estimate) |
| alpha | D22 +24.2° (c = 1.3 in chloroform) |
| Density | 0.9506 (rough estimate) |
| refractive index | 1.5250 (estimate) |
| storage temp. | -20°C |
| solubility | chloroform: 0.1 g/mL, clear, colorless |
| pka | 15.14±0.70(Predicted) |
| form | A solid |
| color | White to off-white |
| Water Solubility | Insoluble in water. |
| Merck | 14,2200 |
| BRN | 2418594 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 80-97-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Cholestanol(80-97-7) |
| EPA Substance Registry System | Dihydrocholesterol (80-97-7) |
Safety information for Dihydrocholesterol
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P281:Use personal protective equipment as required. P311:Call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dihydrocholesterol
Dihydrocholesterol manufacturer
Sri Neelima Laboratories
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

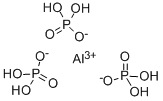
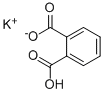
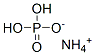
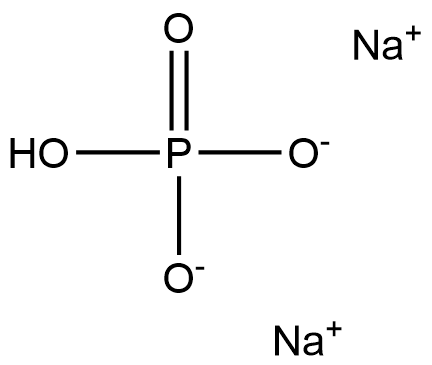
You may like
-
 80-97-7 Dihydrocholesterol 99%View Details
80-97-7 Dihydrocholesterol 99%View Details
80-97-7 -
 5alpha-Cholestan-3beta-ol CAS 80-97-7View Details
5alpha-Cholestan-3beta-ol CAS 80-97-7View Details
80-97-7 -
 β-Cholestanol (contains α-Cholestanol) CAS 80-97-7View Details
β-Cholestanol (contains α-Cholestanol) CAS 80-97-7View Details
80-97-7 -
 Dihydrocholesterol CAS 80-97-7View Details
Dihydrocholesterol CAS 80-97-7View Details
80-97-7 -
 Cholestanol CAS 80-97-7View Details
Cholestanol CAS 80-97-7View Details
80-97-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8