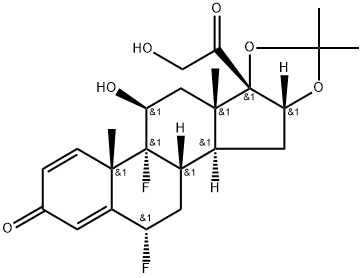
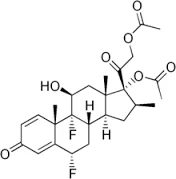
Diflorasone diacetate
Synonym(s):6α,9-Difluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-diacetate
- CAS NO.:33564-31-7
- Empirical Formula: C26H32F2O7
- Molecular Weight: 494.52
- MDL number: MFCD00079159
- EINECS: 251-575-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-28 16:00:23
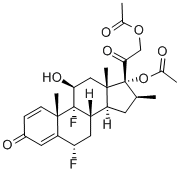
What is Diflorasone diacetate?
Chemical properties
White Solid
Originator
Florone,Upjohn,US,1978
The Uses of Diflorasone diacetate
An anti-inflammatory and anti-itching corticosteroid usually present in topical creams.
The Uses of Diflorasone diacetate
Anti-inflammatory (topical)
Definition
ChEBI: The 17,21-diacetate derivative of diflorasone. It is used topically for its anti-inflammatory and antipruritic properties in the treatment of various skin disorders.
Manufacturing Process
6α-Fluoro-9β-epoxy-17α,21-dihydroxy-16α-methyl-1,4-pregnadiene-3,20-
dione-21-acetate:To a solution of 6.78 g of 6α-fluoro-9α-bromo-11β,17α,21-
trihydroxy-16α-methyl-1,4-pregnadiene-3,20-dione-21-acetate in 175 ml of
acetone was added 6.78 g of potassium acetate and the resulting suspension
was heated under reflux for a period of 17 hours. The mixture was then
concentrated to approximately 60 ml volume at reduced pressure on the
steam bath, diluted with water and extracted with methylene chloride. The
methylene chloride extracts were combined, washed with water, dried over
anhydrous sodium sulfate and evaporated. The residue was redissolved in
methylene chloride and chromatographed over 500 g of Florisil anhydrous
magnesium silicate. The column was eluted with 1 liter portions of hexanes
(Skellysolve B) containing increasing proportions of acetone. There was so
eluted 6α-fluoro-9β,11β-epoxy-16α-methyl-17α,21-dihydroxy-1,4-
pregnadiene-3,20-dione-21-acetate which was freed of solvent by evaporation
of the eluates.
6α,9α-Difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20-
dione-2-1-acetate: To approximately 1.3 g of hydrogen fluoride contained in a
polyethylene bottle and maintained at -60C was added 2.3 ml of
tetrahydrofuran and then a solution of 500 mg (0,0012 mol) of 6α-fluoro9β,11β-epoxy-16α-methyl-17α,21-dihydroxy-1,4-pregnadiene-3,20-dione-21-
acetate in two ml of methylene chloride. The steroid solution was rinsed in
with an additional 1 ml of methylene chloride. The light red colored solution
was then kept at approximately -30°C for 1 hour and at -10°C for 2 hours. At
the end of this period it was mixed cautiously with an excess of cold sodium
bicarbonate solution and the organic material extracted with the aid of
additional methylene chloride.
The combined extracts were washed with water, dried over anhydrous sodium
sulfate and concentrated to approximately 35 ml. The solution was
chromatographed over 130 g of Florisil anhydrous magnesium silicate. The
column was developed with 260 ml portions of hexanes (Skellysolve B)
containing increasing proportions of acetone. There was thus eluted 6α,9αdifluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20-dione-21-
acetate which was freed of solvent by evaporation of the eluate fractions.
6α,9α-Difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20-
dione: 3.25 g of 6α,9α-difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-
pregnadiene-3,20-dione-21-acetate was dissolved in 325 ml of methanol,
previously purged of air-oxygen by passing nitrogen through it for 10 minutes
and thereto was added a solution of 1.63 g of potassium bicarbonate in 30 ml
of water, similarly purged of oxygen. The mixture was allowed to stand at
room temperature for a period of 5 hours in a nitrogen atmosphere,
thereupon neutralized with 2.14 ml of acetic acid in 40 ml of water. The
mixture was concentrated to approximately one-third volume at reduced
pressure on a 60°C water bath. Thereupon 250 ml of water was added and
the mixture chilled. The crystalline product was collected on a filter, washed
with water and dried to give 6α,9α-difluoro-11β,17α,21-trihydroxy-16αmethyl-1,4-pregnadiene-3,20-dione.
The diflorasone is reacted with orthoacetic acid trimethyl ester in the presence
of toluenesulfonic acid to give diflorasone diacetate.
brand name
Florone (Pharmacia & Upjohn); Psorcon (Pharmacia & Upjohn); Psorcon (Sanofi Aventis).
Therapeutic Function
Antiinflammatory
Properties of Diflorasone diacetate
| Melting point: | 221-223° (dec) |
| Boiling point: | 235°C (rough estimate) |
| alpha | D +61° (chloroform) |
| Density | 1.2001 (estimate) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 12.63±0.70(Predicted) |
| form | Solid |
| form | neat |
| color | White to Off-White |
| Water Solubility | 6.5mg/L(22 ºC) |
| CAS DataBase Reference | 33564-31-7(CAS DataBase Reference) |
Safety information for Diflorasone diacetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Diflorasone diacetate
Diflorasone diacetate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 33564-31-7 Diflorasone diacetate 98%View Details
33564-31-7 Diflorasone diacetate 98%View Details
33564-31-7 -
 Diflorasone diacetate CAS 33564-31-7View Details
Diflorasone diacetate CAS 33564-31-7View Details
33564-31-7 -
 Diflorasone Diacetate Usp 2557-49-5 Api Bulk DrugsView Details
Diflorasone Diacetate Usp 2557-49-5 Api Bulk DrugsView Details
33564-31-7 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
