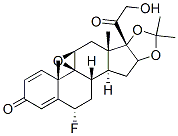
Fluocinolone Acetonide
Synonym(s):Fluocinolone acetonide;Sinalar;Synandone;6α,9α-Difluoro-11β,16α,17α,21-tetrahydroxy-1,4-pregnadiene-3,20-dione;6α,9α-Difluoro-16α-hydroxyprednisolone 16,17-acetonide
- CAS NO.:67-73-2
- Empirical Formula: C24H30F2O6
- Molecular Weight: 452.49
- MDL number: MFCD00010525
- EINECS: 200-668-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 20:43:22
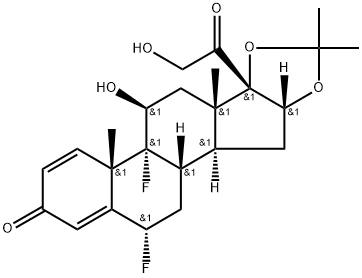
What is Fluocinolone Acetonide?
Absorption
When administered as an eye implant, fluocinolone acetonide presents a sustained delivery for even 12 months in which there can be observed a sustained release. The concentration of fluocinolone acetonide are generally higher in the vitreous and retina with a little dispersion to the aqueous humor.
There are reports indicating that topical administration of fluocinolone acetonide produces a percutaneous absorption which is determined by the vehicle, integrity of the epidermal barrier and the use of occlusive dressing.
Independently of the route of administration, the systemic absorption of fluocinolone acetonide is below 0.1 ng/ml which indicates that the systemic distribution is very minimal and the effect of fluocinolone is mainly local.
Toxicity
Studies to determine the carcinogenic and its effect in fertility have not been performed. It is important to consider that several corticosteroids have been shown to present genotoxic potential but fluocinolone acetonide was shown to not be genotoxic in the Ames test and mouse lymphoma TK assay.
Chemical properties
Crystalline Solid
Originator
Synalar,Syntex,US,1961
The Uses of Fluocinolone Acetonide
Fluocinolone acetonide is primarily used in dermatology to reduce skin inflammation and relieve itching. It acts as an inhibitor of tumor cells and it can regulate lipid metabolism by modulating gene expression. It can also inhibit the expression of VEGF (vascular endothelial growth factor) in the retinal pigment epithelial cell line due to its high glucocorticoid receptor affinity. Furthermore, it can inhibit TNF-α angiogenesis.
The Uses of Fluocinolone Acetonide
Fluocinolone acetonide (Derma-Smoothe/FS, Flurosyn, Capex, Synalar) is a synthetic fluorinated corticosteroid.
The Uses of Fluocinolone Acetonide
A synthetic glucocorticoid VEGF inhibitor; anti-inflammatory.
Background
Fluocinolone acetonide, with the formula 6-alpha, 9-alpha-difluoro-16-alpha, 17 alpha-acetonide, is a corticosteroid that presents a high lipophilicity. It has been used extensively in dermatological preparations and it has also been investigated thoroughly for its use in implantable corticosteroid devices. This type of device containing fluocinolone acetonide was developed by Taro Pharmaceuticals and approved by FDA in May 2016.
What are the applications of Application
Fluocinolone Acetonide is a synthetic glucocorticoid VEGF inhibitor
Indications
Fluocinolone acetonide has been used extensively in different medical areas.
-In dermatology, it is extensively used for the relief of inflammatory dermatosis, dermatitis, psoriasis, hypertrophic tissues, keloid tissues and atopic dermatitis.
-It has been used in shampoo products as a low to medium potency corticosteroid for the treatment of seborrheic dermatitis of the scalp.
-In ear drops, it is used as a low to medium potency corticosteroid for the treatment of chronic eczematous external otitis in adults and pediatric patients 2 years and older.
-As an intravitreal implant, it is indicated for the treatment of diabetic macular edema with patients that have been previously treated with a course of corticosteroids and no clinically significant rise in intraocular pressure.
-Fluocinolone acetonide was announced on October 15, 2018 to be FDA approved for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye.
-Some reports have indicated the use of fluocinolone acetonide as a vasoprotective agent and for its use in the treatment of first-degree hemorrhoids.
Definition
ChEBI: A fluorinated steroid that is flunisolide in which the hydrogen at position 9 is replaced by fluorine. A corticosteroid with glucocorticoid activity, it is used (both as the anhydrous form and as the dihydrate) in creams, gels and ointments for the treatme t of various skin disorders.
Indications
Fluocinolone acetonide (Derma-Smoothe/FS, Flurosyn, Capex, Synalar) is a synthetic fluorinated corticosteroid.
Manufacturing Process
A mixture of 1.2 grams of 6α-fluoro-16α-hydroxy-hydrocortisone, 4 cc of
acetic anhydride and 8 cc of pyridine was heated at 60°C for 2 hours and then
kept at room temperature for 2 hours. Ice and water were added and the
solid was collected, washed with water, dried and recrystallized from
methylene chloride-methanol, thus giving 1.05 grams of the 16,21-diacetate
of 6α-fluoro-16α-hydroxy-hydrocortisone (solvated) of MP 182° to 187°C;concentration of the mother liquors afforded an additional 130 mg of the same
compound, MP 184° to 187°C. By recrystallization from the same solvents
there was obtained the compound with a lower constant melting point of 175°
to 177°C.
2.94 grams of the 16,21-diacetate of 6α-fluoro-16α-hydroxy-hydrocortisone
was mixed with 60 cc of dimethylformamide, 3.6 cc of pyridine and 2.4 cc of
methane-sulfonyl chloride was heated on the steam bath for 2 hours. The
diacetate of 6α-fluoro-16α-hydroxy-hydrocortisone had been prepared as set
forth above, and further dried by azeotropic distillation with benzene; the
dimethylformamide had been previously distilled. After the 2 hours on the
steam bath the mixture was cooled and poured into saturated aqueous
sodium bicarbonate solution; the product was extracted with methylene
chloride, the extract was washed with water, dried over anhydrous sodium
sulfate and the solvent was evaporated.
The residue was chromatographed on 90 grams of silica gel eluting the
product with methylene chloride-acetone (9:1) and then recrystallizing from
methylene chloride-methanol. There was thus obtained 1.6 grams of the
16,21-diacetate of 6α-fluoro-δ4,9(11)-pregnadiene-16α,-17α,21-triol-3,20-dione
with MP 110° to 114°C; the analytical sample melted at 115° to 117°C,
[α]D+23.5° (chloroform), λ max. 234 to 236 nm, log ε 4.18.
A mixture of 1.38 grams of the above compound and 15 cc of dioxane was
treated with 1.9 cc of a 0.5 N aqueous solution of perchloric acid and 600 mg
of N-bromoacetamide, adding the latter in the dark, in three portions, in the
course of half an hour and under continuous stirring, It was then stirred for a
further 1% hours in the dark, then the excess of reagent was decomposed by
the addition of aqueous sodium bisulfite solution and ice water was added; the
product was extracted with methylene chloride, washed with water, dried over
anhydrous sodium sulfate and the solvent was evaporated under reduced
pressure, thus giving a yellow oil consisting of the 16,21-diacetate of 6α-
fluoro-9α-bromo-16α-hydroxy-hydrocortisone which was used for the next
step without further purification.
The above crude bromohydrin was mixed with 2.5 grams of potassium acetate
and 60 cc of acetone and refluxed for 6 hours, at the end of which the
acetone was distilled, water was added to the residue and the product was
extracted with methylene chloride. The extract was washed with water, dried
over anhydrous sodium sulfate and the solvent was evaporated.
Recrystallization of the residue from methanol furnished 800 mg of the 16,21-
diacetate of 6α-fluoro-9β,11β-oxido-δ4-pregnene-16α,17α,21-triol-3,20-dione
with MP 120° to 124°C; by chromatography of the mother liquors on silica gel
there was obtained 180 milligrams more of the same compound with MP 117°
to 119°C. The analytical sample was obtained by recrystallization from
methanol; it showed MP 125° to 127°C.
To a solution of 1.6 grams of anhydrous hydrogen fluoride in 2.85 grams of
tetrahydrofurane and 10 cc of methylene chloride cooled to -60°C was added
a solution of 650 mg of the 16,21-diacetate of 6α-fluoro-9β,11β-oxido-δ4-
pregnene-16α,17α,21-triol-3,20-dione in 20 cc of methylene chloride and the
mixture was kept at -10°C for 72 hours. It was then poured into saturated
aqueous sodium bicarbonate solution and the organic layer was separated,
washed with water, dried over anhydrous sodium sulfate and evaporated. The residue was reacetylated by heating with 3 cc of acetic anhydride and 6 cc of
pyridine for 1 hour on the steam bath. The reagents were evaporated under
reduced pressure and the residue was chromatographed on 30 grams of silica
gel. Upon elution with methylene chloride-acetone (9:1) and recrystallization
of the residue from methylene chloride-methanol there was obtained 290 mg
of the 16,21-diacetate of 6α,9α-difluoro-16α-hydroxy-hydrocortisone which
melted with loss of solvent at 140° to 150°C. Recrystallization from acetonehexane
afforded the analytical sample which was dried at 130°C; it then
showed a MP of 182° to 185°C.
brand name
Fluonid (Allergan); Fluotrex (Savage); FS Shampoo (Galderma); Retisert (Bausch & Lomb); Synalar (Medicis).
Therapeutic Function
Glucocorticoid, Antiinflammatory
General Description
Fluocinolone acetonide,6α,9-difluoro-11β,21-dihydroxy-16α,17-[(1-methylethylidene)bis(oxy)]pregna-1,4-diene-3,20-dione, also known as6α-fluorotriamcinolone acetonide, is the 21-acetate derivativeof fluocinolone acetonide and is about 5 times morepotent than fluocinolone acetonide in at least one topicalactivity assay.
Pharmacokinetics
Fluocinolone acetonide is a synthetic anti-inflammatory corticosteroid and thus, the effect of its interaction with the body produces vasoconstriction and suppression of membrane permeability, mitotic activity, immune response and release of inflammatory mediators.
For its ophthalmic indications, fluocinolone acetonide is administered as intravitreal micro-insert. This preparation was observed in clinical trials to reduce the recurrence of uveitis flares by 2 fold when compared with the non treated patients even after six months after initial administration. As well the intraocular pressure seemed to increase slightly with the presence of the fluocinolone implant but it is important to monitor intraocular pressure.
Metabolism
Following absorption, fluocinolone acetonide metabolism is primarily hepatic. It is important to mention that the systemically absorbed dose is very minimal.
Properties of Fluocinolone Acetonide
| Melting point: | 267-269 °C(lit.) |
| Boiling point: | 578.5±50.0 °C(Predicted) |
| alpha | D +95° (chloroform) |
| Density | 1.1826 (estimate) |
| refractive index | 103 ° (C=1, MeOH) |
| storage temp. | Refrigerator |
| solubility | Practically insoluble in water, soluble in acetone and in ethanol |
| form | neat |
| pka | 12.78±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Soluble in water (partly), DMSO, alcohol, chloroform and methanol. |
| Merck | 14,4150 |
| CAS DataBase Reference | 67-73-2(CAS DataBase Reference) |
| EPA Substance Registry System | Fluocinolone acetonide (67-73-2) |
Safety information for Fluocinolone Acetonide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Fluocinolone Acetonide
| InChIKey | FEBLZLNTKCEFIT-VSXGLTOVSA-N |
Fluocinolone Acetonide manufacturer
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
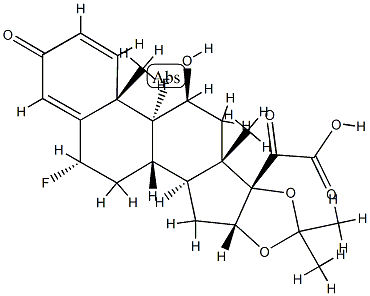
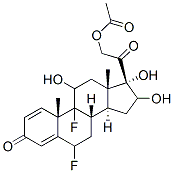
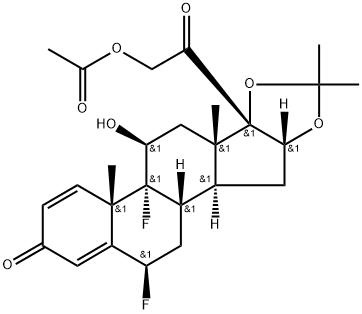
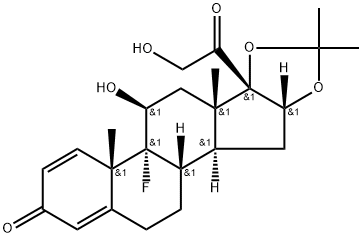
![(6α,11β,16α)-6,9-Difluoro-11,21-dihydroxy-21-Methoxy-16,17-[(1-Methylethylidene)bis(oxy)]-pregna-1,4-diene-3,20-dione](https://img.chemicalbook.in/CAS/20180808/GIF/62013-83-6.gif)

You may like
-
 Fluocinolone Acetonide 98%View Details
Fluocinolone Acetonide 98%View Details -
 Fluocinolone Acetonide 99%View Details
Fluocinolone Acetonide 99%View Details -
 Fluocinolone acetonide 99%View Details
Fluocinolone acetonide 99%View Details -
 67-73-2 99%View Details
67-73-2 99%View Details
67-73-2 -
 Fluocinolone acetonide 96% CAS 67-73-2View Details
Fluocinolone acetonide 96% CAS 67-73-2View Details
67-73-2 -
 Fluocinolone acetonide CAS 67-73-2View Details
Fluocinolone acetonide CAS 67-73-2View Details
67-73-2 -
 67-73-2 Fluocinolone Acetonide Powder, DrumView Details
67-73-2 Fluocinolone Acetonide Powder, DrumView Details
67-73-2 -
 Fluocinolone Acetonide PowderView Details
Fluocinolone Acetonide PowderView Details
67-73-2
