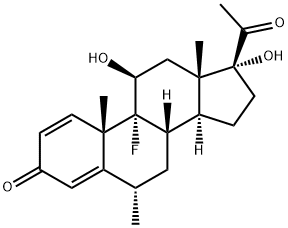
Diflorasone
- CAS NO.:2557-49-5
- Empirical Formula: C22H28F2O5
- Molecular Weight: 410.46
- MDL number: MFCD00200358
- EINECS: 219-875-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-21 18:03:03
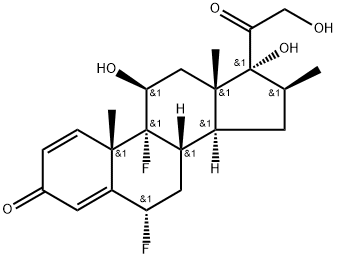
What is Diflorasone?
Absorption
Topical corticosteroids can be absorbed from intact healthy skin. The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusion, inflammation and/or other disease processes in the skin may also increase percutaneous absorption.
Toxicity
Topically applied diflorasone can be absorbed in sufficient amounts to produce systemic effects. Symptoms of overdose include thinning of skin and suppression of adrenal cortex (decreased ability to respond to stress).
Chemical properties
Off-White Solid
Originator
Florone,Upjohn,US,1978
The Uses of Diflorasone
An anti-inflammatory and anti-itching corticosteroid usually present in topical creams.
Indications
For relief of the inflammatory and pruritic manifestations of corticosteroid responsive dermatoses.
What are the applications of Application
Diflorasone is an anti-inflammatory and anti-itching corticosteroid
Background
Diflorasone is a topical corticosteroid used to treat itching and inflammation of the skin.
Definition
ChEBI: The 16beta-analogue of flumethasone. It is used as the 17,21-diacetate as a topical anti-inflammatory and antipruritic in the treatment of various skin disorders.
Manufacturing Process
6α-Fluoro-9β-epoxy-17α,21-dihydroxy-16α-methyl-1,4-pregnadiene-3,20-
dione-21-acetate:To a solution of 6.78 g of 6α-fluoro-9α-bromo-11β,17α,21-
trihydroxy-16α-methyl-1,4-pregnadiene-3,20-dione-21-acetate in 175 ml of
acetone was added 6.78 g of potassium acetate and the resulting suspension
was heated under reflux for a period of 17 hours. The mixture was then
concentrated to approximately 60 ml volume at reduced pressure on the
steam bath, diluted with water and extracted with methylene chloride. The
methylene chloride extracts were combined, washed with water, dried over
anhydrous sodium sulfate and evaporated. The residue was redissolved in
methylene chloride and chromatographed over 500 g of Florisil anhydrous
magnesium silicate. The column was eluted with 1 liter portions of hexanes
(Skellysolve B) containing increasing proportions of acetone. There was so
eluted 6α-fluoro-9β,11β-epoxy-16α-methyl-17α,21-dihydroxy-1,4-
pregnadiene-3,20-dione-21-acetate which was freed of solvent by evaporation
of the eluates.
6α,9α-Difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20-
dione-2-1-acetate: To approximately 1.3 g of hydrogen fluoride contained in a
polyethylene bottle and maintained at -60C was added 2.3 ml of
tetrahydrofuran and then a solution of 500 mg (0,0012 mol) of 6α-fluoro9β,11β-epoxy-16α-methyl-17α,21-dihydroxy-1,4-pregnadiene-3,20-dione-21-
acetate in two ml of methylene chloride. The steroid solution was rinsed in
with an additional 1 ml of methylene chloride. The light red colored solution
was then kept at approximately -30°C for 1 hour and at -10°C for 2 hours. At
the end of this period it was mixed cautiously with an excess of cold sodium
bicarbonate solution and the organic material extracted with the aid of
additional methylene chloride.
The combined extracts were washed with water, dried over anhydrous sodium
sulfate and concentrated to approximately 35 ml. The solution was
chromatographed over 130 g of Florisil anhydrous magnesium silicate. The
column was developed with 260 ml portions of hexanes (Skellysolve B)
containing increasing proportions of acetone. There was thus eluted 6α,9αdifluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20-dione-21-
acetate which was freed of solvent by evaporation of the eluate fractions.
6α,9α-Difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-pregnadiene-3,20-
dione: 3.25 g of 6α,9α-difluoro-11β,17α,21-trihydroxy-16α-methyl-1,4-
pregnadiene-3,20-dione-21-acetate was dissolved in 325 ml of methanol,
previously purged of air-oxygen by passing nitrogen through it for 10 minutes
and thereto was added a solution of 1.63 g of potassium bicarbonate in 30 ml
of water, similarly purged of oxygen. The mixture was allowed to stand at
room temperature for a period of 5 hours in a nitrogen atmosphere,
thereupon neutralized with 2.14 ml of acetic acid in 40 ml of water. The
mixture was concentrated to approximately one-third volume at reduced
pressure on a 60°C water bath. Thereupon 250 ml of water was added and
the mixture chilled. The crystalline product was collected on a filter, washed
with water and dried to give 6α,9α-difluoro-11β,17α,21-trihydroxy-16αmethyl-1,4-pregnadiene-3,20-dione.
The diflorasone is reacted with orthoacetic acid trimethyl ester in the presence
of toluenesulfonic acid to give diflorasone diacetate.
brand name
Florone (Pharmacia & Upjohn); Psorcon (Pharmacia & Upjohn); Psorcon (Sanofi Aventis).
Therapeutic Function
Antiinflammatory
Pharmacokinetics
Like other topical corticosteroids, diflorasone has anti-inflammatory, antipruritic, and vasoconstrictive properties. Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Diflorasone is a potent topical corticosteroid that should not be used with occlusive dressings. It is recommended that treatment should be limited to 2 consecutive weeks and therapy should be discontinued when adequate results have been achieved.
Metabolism
Metabolized, primarily in the liver, and then excreted by the kidneys.
Properties of Diflorasone
| Melting point: | 228-239°C |
| Boiling point: | 569.8±50.0 °C(Predicted) |
| Density | 1.36±0.1 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Methanol (Slightly) |
| form | Solid |
| pka | 11.98±0.70(Predicted) |
| color | Off-White to Pale Yellow |
| CAS DataBase Reference | 2557-49-5 |
Safety information for Diflorasone
Computed Descriptors for Diflorasone
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Diflorasone 98% (HPLC) CAS 2557-49-5View Details
Diflorasone 98% (HPLC) CAS 2557-49-5View Details
2557-49-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1