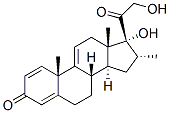
Desoximetasone
- CAS NO.:382-67-2
- Empirical Formula: C22H29FO4
- Molecular Weight: 376.46
- MDL number: MFCD00083301
- EINECS: 206-845-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 18:23:36
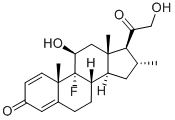
What is Desoximetasone?
Absorption
Topical corticosteroids can be absorbed from intact healthy skin. The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusion, inflammation and/or other disease processes in the skin may also increase percutaneous absorption.
Toxicity
Topically applied desoximetasone can be absorbed in sufficient amounts to produce systemic effects. Symptoms of overdose include thinning of skin and suppression of adrenal cortex (decreased ability to respond to stress).
Chemical properties
Off-White Solid
Originator
Topicorte,Roussel,France,1968
The Uses of Desoximetasone
Glucocorticoid anti-inflammatory. It is also found as an impurity in Dexamethasone (D298800).
What are the applications of Application
Desoximetasone is a glucocorticoid antiinflammatory agent
Indications
For the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
Background
A topical anti-inflammatory glucocorticoid used in dermatoses, skin allergies, psoriasis, etc.
Definition
ChEBI: Dexamethasone in which the hydroxy group at the 17alpha position is substituted by hydrogen. A synthetic corticosteroid with glucocorticoid activity, it is used as an anti-inflammatory and anti-pruritic in the treatment of various skin dis rders, including skin allergies and psoriasis.
Manufacturing Process
(a) Production of 16α-Methyl-4-Pregnene-11β,21-diol-3,20-Dione (= 16αMethylcorticosterone): A fermenter of stainless steel having a 50 liter capacity
is charged with 30 liters of a nutrient solution containing:
sterilized for ? hour at 120°C and after cooling, inoculated with a spore
suspension of Curvularia lunata which is obtained by rinsing a seven day corn
culture (15 grams corn) with approximately 100 cc of physiological sodium
chloride solution.
After two days of culturing at 25°C under stirring (220 revolutions per minute)
and ventilating (1.65 m3/hr), 18 liters of the obtained culture are removed
under sterile conditions and introduced into a fermenter of the same size
charged with 28.2 liters of a nutrient solution containing:
After 24 hours cultivation under stirring and ventilation as described above,
7.5 grams of 16α-methyldesoxycorticosterone, obtained by saponification of
the corresponding 21-acetate and melting at 102-104°C, in 200 cc of ethanol
are added and fermented under the same conditions for 28 hours.
The course of the fermentation is tested by removal of samples, which are
extracted with methyl isobutyl ketone. The extract is analyzed by paper
chromatography in a system of dioxane + toluene/propylene glycol.
After the end of the fermentation (28 hours) the culture broth is filtered off by
suction over a large suction filter. The mycel residue is washed with water
several times. The filtrate is extracted three times, each time with 10 liters of
methyl isobutyl ketone. The extract is concentrated under vacuum in a
circulating evaporator and in a round flask carefully dried under vacuum. The
residue is crystallized from acetone/isopropyl ether. The melting point is 157-
158°C (fermentation yield = 60%). The pure product yield obtained after a
second crystallization and chromatography of the mother liquor on silica gel
amounts to 53% of the theoretical.
(b) 16α-Methyl-9α-Fluoro-?4-Pregnene-11β,21-Diol-3,20-Dione: 7.5 grams of
16α-methyl-9α-fluoro-?4-pregnene-21-ol-3,20-dione-21-acetate, obtained
from Step (a) by acetylating with acetic anhydride in pyridine followed by
reaction with HF in pyridine at 0°C, are fermented for 36 hours with
Curvularia lunata (Mutant NRRL 2380), whereby the 21-acetate group is
simultaneously saponified, and then further worked up. The residue is
extracted with MIBK, subjected to chromatography on silica gel and there is
obtained from chloroform/ethyl acetate (2:1) an eluate containing the 11βhydroxy compound, which is further dehydrogenated as the crude product.
(c) 16α-Methyl-9α-Fluoro-?1,4-Pregnadiene-11β,21-Diol-3,20-Dione: 16αmethyl-9α-fluoro-β4-pregnene-11β,21-diol-3,20-dione obtained as the crude
product under Step (b) above, is fermented with Bacillus lentus for 30 hours
and further worked up. The residue is extracted with methyl isobutyl ketone
and there is obtained as the crude product 16α-methyl-9α-fluoro-?1,4-
pregnadiene-11β,21-diol-3,20-dione.
brand name
Topicort (Taro) [Name previously used: Desoxymethasone.].
Therapeutic Function
Antiinflammatory
General Description
Desoximetasone, 9-fluoro-11β, 21-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione, like clocortolone pivalate, lacks a C17α OH group inits structure.
Pharmacokinetics
Like other topical corticosteroids, desoximetasone has anti-inflammatory, antipruritic, and vasoconstrictive properties. Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Desoximetasone is a potent topical corticosteroid that should not be used with occlusive dressings. It is recommended that treatment should be limited to 2 consecutive weeks and therapy should be discontinued when adequate results have been achieved.
Metabolism
Metabolized, primarily in the liver, and then excreted by the kidneys.
Properties of Desoximetasone
| Melting point: | 213-215°C |
| Boiling point: | 532.3±50.0 °C(Predicted) |
| alpha | D +109° (chloroform) |
| Density | 376.46 |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 12.98±0.10(Predicted) |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 382-67-2(CAS DataBase Reference) |
Safety information for Desoximetasone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Desoximetasone
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
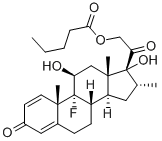
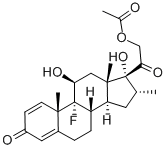
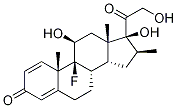
You may like
-
 382-67-2 Desoxymetasone 98%View Details
382-67-2 Desoxymetasone 98%View Details
382-67-2 -
 Desoximetasone 99%View Details
Desoximetasone 99%View Details -
 Desoximetasone 98%View Details
Desoximetasone 98%View Details
382-67-2 -
 Desoximetasone 99%View Details
Desoximetasone 99%View Details -
 Desoximetasone CAS 382-67-2View Details
Desoximetasone CAS 382-67-2View Details
382-67-2 -
 Desoximetasone 98% (HPLC) CAS 382-67-2View Details
Desoximetasone 98% (HPLC) CAS 382-67-2View Details
382-67-2 -
 Desoximetasone Raw Powder Cas No 382-67-2, 1 KgView Details
Desoximetasone Raw Powder Cas No 382-67-2, 1 KgView Details
382-67-2 -
 Desoximetasone API Powder, 1 KgView Details
Desoximetasone API Powder, 1 KgView Details
382-67-2
