Diethylenetriamine
Synonym(s):2,2′-Diaminodiethylamine;2,2′-Iminobis (ethylamine), Bis(2-aminoethyl)amine, 2,2′-Diaminodiethylamine;2,2′-Iminodiethylamine;Bis(2-aminoethyl)amine;Diethylenetriamine
- CAS NO.:111-40-0
- Empirical Formula: C4H13N3
- Molecular Weight: 103.17
- MDL number: MFCD00008171
- EINECS: 203-865-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
What is Diethylenetriamine?
Description
Diethylenetriamine is a hardener in epoxy resins of the Bisphenol A type. It has been reported as a sensitizer in ultrasonic baths for cleaning jewels, in synthetic lubricants and in carbonless copy paper.
Chemical properties
Diethylenetriamine is a corrosive yellow liquid and a solvent. It has a strong ammonia odor and is prone to absorbing moisture. It can dissolve in water, acetone, benzene, ether, methanol, but is not soluble in n-heptane.
The Uses of Diethylenetriamine
Diethylenetriamine is manufactured by reacting ethylene dichloride and ammonia. It is used as a solvent, in organic syntheses, and in a variety of industrial applications including use as a fuel component.
The Uses of Diethylenetriamine
Diethylenetriamine is a solvent for sulfur, acidic gas, resin and dye intermediates for organic synthesis; saponification agent for acidic materials; fuel component; hardener for epoxy resins.
Production Methods
Diethylenetriamine is produced by the reaction of ethylene dichloride with ammonia. Diethylenetriamine is used in biological studies, for polyamines inhibition to carbonic anhydrases by anchoring to the zinc-coordinated water molecule.
Definition
ChEBI: Diethylenetriamine is a triamine and a polyazaalkane.
Synthesis Reference(s)
Journal of the American Chemical Society, 105, p. 5002, 1983 DOI: 10.1021/ja00353a025
General Description
A yellow liquid with an ammonia-like odor. Less dense than water. Corrosive to metals and tissue. Vapors heavier than air. Burns, although possibly difficult to igntie. Toxic oxides of nitrogen produced during combustion. Used as a solvent for plastics and dyes and in chemical synthesis.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Diethylenetriamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Health Hazard
Prolonged breathing of vapors may cause asthma. Liquid burns skin and eyes. A skin rash can form.
Health Hazard
Brief contact with concentrated diethylenetriamine can produce severe local injury to the eyes and skin resembling the effect from strong base. Human subjects are susceptible to sensitization responses either as dermatitis or an asthma-like response. A time-weighted average of 1 p.p.m. is recommenced for diethylenetriamine (ACGIH 1986).
Fire Hazard
Special Hazards of Combustion Products: Irritating vapors are generated when heated.
Flammability and Explosibility
Non flammable
Industrial uses
Diethylenetriamine is used as an intermediate in the production of reactive polyamide resins, and in the production of aminoamides and imidazolines from fatty acids. It is also used in the production of paper wet strength resins and piperazine. Diethylenetriamine serves as a solvent for sulfur, acid gases, resins and dyes (HSDB 1989).
Contact allergens
Diethylenetriamine is a hardener in epoxy resins of the Bisphenol A type. It has been reported to be a sensitizer when used in an ultrasonic bath for cleaning jewels, in synthetic lubricants, or in carbonless copy paper.
Safety Profile
Poison by skin contact and intraperitoneal routes. Moderately toxic by ingestion. Corrosive. A severe skin and eye irritant. High concentration of vapors causes irritation of respiratory tract, nausea, and vomiting. Repeated exposures can cause asthma and sensitization of skin. Combus uble when exposed to heat or flame; can react with oxidizing materials. Mxture with nitromethane is a shock-sensitive explosive. Ignites on contact with cellulose nitrate of high surface area. To fight fire, use alcohol foam. When heated to decomposition it emits toxic fumes of NOx. See also AMINES.
Potential Exposure
PrimaryIrritant. This material is used in textile finishes, fungicides,corrosion inhibitors, adhesives, asphalt additives, as a solvent for sulfur, acid gases, resins, and dyes.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting.
Carcinogenicity
DETA has a strong ammonia-like odor,
but it does not provide adequate warning of
hazardous concentrations.
The 2003 ACGIH threshold limit valuetime-
weighted average (TLV-TWA) for diethylene
triamine is 1ppm (4.2mg/m3) with a
notation for skin absorption.
Metabolism
Diethylenetriamine is readily absorbed through the gastrointestinal tract and 96% of the administered dose is excreted within 48 h (USEPA 1983a). Roughly equal amounts are excreted in the feces and urine with at least 4 metabolites being detected (but not identified) in the latter. Only a small proportion (<2%) was recovered as expired carbon dioxide. Any residual remaining in the animal was found primarily in kidney, liver, bladder and large intestine.
Storage
Color Code—White: Corrosive or Contact Hazard;Store separately in a corrosion-resistant location. Prior to working with this chemical you should be trained on itsproper handling and storage. Before entering confined spacewhere this chemical may be present, check to make surethat an explosive concentration does not exist. Store intightly closed containers in a cool, well-ventilated areaaway from acids, halogenated organics, and oxidizers (suchas perchlorates, peroxides, chlorates, nitrates, and permanganates). Protect containers from physical damage. Sourcesof ignition, such as smoking and open flames, are prohibited where this chemical is used, handled, or stored in amanner that could create a potential fire or explosion hazard. Metal containers involving the transfer of=gallons ormore of this chemical should be grounded and bonded.Drums must be equipped with self-closing valves, pressurevacuum bungs, and flame arresters. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of this chemical.
Shipping
This material requires a “CORROSIVE” label. Itfalls in Hazard Class 8 and Packing Group II.
Purification Methods
Dry the amine with Na and distil, preferably under reduced pressure, or in a stream of N2. [Beilstein 4 IV 1284.]
Incompatibilities
Forms explosive mixture with air. Ignitesspontaneously on contact with cellulose nitrate. Contact withsilver, cobalt, or chromium compounds may cause explosions.Incompatible with acids, halogenated organics, organic anhydrides, isocyanates, vinyl acetate, acrylates, substituted allyls,alkylene oxides, epichlorohydrin, ketones, aldehydes, alcohols, glycols, mercury, phenols, cresols, caprolactum solution,strong oxidizers. Attacks aluminum, copper, copper alloys,lead, tin, zinc and alloys.
Properties of Diethylenetriamine
| Melting point: | -40 °C |
| Boiling point: | 206 °C |
| Density | 0.955 g/mL at 25 °C(lit.) |
| vapor density | 3.6 (vs air) |
| vapor pressure | 0.08 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 90 °C |
| storage temp. | Store below +30°C. |
| solubility | miscible |
| solubility | Chloroform (Soluble), Methanol (Slightly) |
| pka | pK1:4.42(+3);pK2:9.21(+2);pK3:10.02(+1) (25°C) |
| appearance | colorless to yellow liquid |
| form | Liquid |
| color | Clear |
| Odor | Strong ammoniacal; mildly ammoniacal. |
| PH | >12 (100g/l, H2O, 20℃) |
| explosive limit | 1-10%(V) |
| Water Solubility | miscible |
| Sensitive | Air Sensitive |
| BRN | 605314 |
| Exposure limits | ACGIH: TWA 1 ppm (Skin) NIOSH: TWA 1 ppm(4 mg/m3) |
| Stability: | Stable, but absorbs carbon dioxide from the air. Incompatible with strong oxidizing agents, copper and its alloys. |
| CAS DataBase Reference | 111-40-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,2-Ethanediamine, N-(2-aminoethyl)-(111-40-0) |
| EPA Substance Registry System | Diethylenetriamine (111-40-0) |
Safety information for Diethylenetriamine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H317:Sensitisation, Skin H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Diethylenetriamine
| InChIKey | RPNUMPOLZDHAAY-UHFFFAOYSA-N |
Diethylenetriamine manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
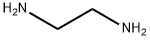

You may like
-
 Di Ethylene Tri Amine (DETA) 99%View Details
Di Ethylene Tri Amine (DETA) 99%View Details -
 DIETHYLENE TRIAMINE (DETA) 99%View Details
DIETHYLENE TRIAMINE (DETA) 99%View Details -
 Diethylenetriamine CAS 111-40-0View Details
Diethylenetriamine CAS 111-40-0View Details
111-40-0 -
 Diethylenetriamine CASView Details
Diethylenetriamine CASView Details -
 Di Ethylene Triamine (DETA)View Details
Di Ethylene Triamine (DETA)View Details
111-40-0 -
 DiethylenetriamineView Details
DiethylenetriamineView Details
111-40-0 -
 Diethylene Triamine (DETA)View Details
Diethylene Triamine (DETA)View Details
111-40-0 -
 A3 Diethylenetriamine LiquidView Details
A3 Diethylenetriamine LiquidView Details
111-40-0
