111-40-0
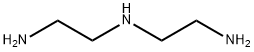
Product Name:
Diethylenetriamine
Formula:
C4H13N3
Synonyms:
2,2′-Diaminodiethylamine;2,2′-Iminobis (ethylamine), Bis(2-aminoethyl)amine, 2,2′-Diaminodiethylamine;2,2′-Iminodiethylamine;Bis(2-aminoethyl)amine;Diethylenetriamine
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Diethylenetriamine appears as a yellow liquid with an ammonia-like odor. Less dense than water. Corrosive to metals and tissue. Vapors heavier than air. Burns, although possibly difficult to igntie. Toxic oxides of nitrogen produced during combustion. Used as a solvent for plastics and dyes and in chemical synthesis. |
|---|---|
| Color/Form | YELLOW LIQUID |
| Odor | AMMONIACAL ODOR |
| Boiling Point | 405 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -38 °F (NTP, 1992) |
| Flash Point | 215 °F (NTP, 1992) |
| Solubility | Very soluble (NTP, 1992) |
| Density | 0.954 at 68 °F (USCG, 1999) - Less dense than water; will float |
| Vapor Density | 3.48 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 0.22 mmHg at 68 °F (NTP, 1992) |
| LogP | -1.3 |
| Autoignition Temperature | 676 °F (USCG, 1999) |
| Viscosity | 0.0714 POISE @ 20 °C |
| Heat of Vaporization | 456.4 J/g @ 0.1 MPa |
| pH | STRONGLY ALKALINE |
| Surface Tension | 43.8 mN/m @ 25 °C |
| Odor Threshold | 10 ppm |
| Refractive Index | INDEX OF REFRACTION: 1.4810 @ 25 °C/D |
| Dissociation Constants | Kb = 7.07X10-5 |
| Kovats Retention Index | 1025 1028 1030 |
| Other Experimental Properties | CORROSIVE TO COPPER & ITS ALLOYS |
| Chemical Classes | Nitrogen Compounds -> Amines, Aliphatic |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H317:Sensitisation, Skin H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 103.17 g/mol |
|---|---|
| XLogP3 | -2.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 103.110947427 g/mol |
| Monoisotopic Mass | 103.110947427 g/mol |
| Topological Polar Surface Area | 64.1 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 26.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
The three nitrogen atoms make diethylenetriamine (DETA) an excellent complexing agent for metals such as cobalt, copper, and platinum. Some DETA-based metal complexes can be used to catalyze organic reactions; examples are the oxidation of phenol with hydrogen peroxide and Heck cross-coupling reactions.