Dicyclohexylammonium nitrite
Synonym(s):Dicyclohexylamine nitrite
- CAS NO.:3129-91-7
- Empirical Formula: C12H24N2O2
- Molecular Weight: 228.33
- MDL number: MFCD00013128
- EINECS: 221-515-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
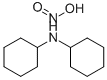
What is Dicyclohexylammonium nitrite?
Description
Dicyclohexylamine nitrite is a flammable whitepowder which has some volatility at room temperature andhigher. Molecular weight=228.38; Melting point=181℃;Flash point=185℃. Partly soluble in water.
Chemical properties
Dicyclohexylamine nitrite is a flammable white powder which has some volatility at room temperature and higher
General Description
A white to light-colored crystalline solid. Insoluble in water and denser than water. Contact may cause irritation to skin, eyes, and mucous membranes. May be toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Dicyclohexylammonium nitrite may be sensitive to prolonged exposure to air. . Insoluble in water.
Reactivity Profile
An oxidizing agent but can serve as a reducing agent. If mixed with reducing agents, including hydrides, sulfides and nitrides, may begin a vigorous reaction that culminates in a detonation.
Health Hazard
Fire may produce irritating and/or toxic gases. Contact may cause burns to skin and eyes. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by friction, heat, sparks or flames. Some may burn rapidly with flare burning effect. Powders, dusts, shavings, borings, turnings or cuttings may explode or burn with explosive violence. Substance may be transported in a molten form at a temperature that may be above its flash point. May re-ignite after fire is extinguished.
Safety Profile
Poison by ingestion and subcutaneous routes. Questionable carcinogen with experimental tumorigenic data. A flammable liquid. When heated to decomposition it emits very toxic fumes of HN02 and NOx. See also NITRITES.
Potential Exposure
It is used as a vapor-phase corrosion inhibitor whereby it vaporizes either from the solid state or from solution, and offers protection against atmospheric rusting. Wrapping paper, plastic wraps; and other materials may be impregnated with dichan to protect metal parts during packaging and storage
First aid
Skin Contact: Flood all areas of body thathave contacted the substance with water. Do not wait toremove contaminated clothing; do it under the water stream.Use soap to help assure removal. Isolate contaminatedclothing when removed to prevent contact by others.Eye Contact: Remove any contact lenses at once. Immediatelyflush eyes well with copious quantities of water or normalsaline for at least 20-30 min. Seek medical attention.Inhalation: Leave contaminated area immediately; breathefresh air. Proper respiratory protection must be supplied toany rescuers. If coughing, difficult breathing, or any othersymptoms develop, seek medical attention at once, even ifsymptoms develop many hours after exposure.Ingestion: Contact a physician, hospital, or poison center atonce. If the victim is unconscious or convulsing, do notinduce vomiting or give anything by mouth. Assure that thepatient’s airway is open and lay him on his side with hishead lower than his body and transport immediately to amedical facility. If conscious and not convulsing, give aglass of water to dilute the substance. Vomiting should notbe induced without a physician’s advice.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobinemia in urine.
storage
Color Code—Red: Flammability Hazard: Store ina flammable materials storage area. Prior to working withthis chemical you should be trained on its proper handlingand storage. Store in a refrigerator under an inert atmosphere for long-term storage.
Shipping
UN2687 Dicyclohexylammonium nitrite, Hazard Class: 4.1; Labels: 4.1-Flammable solid.
Incompatibilities
Highly flammable and a strong oxidizer. Contact with reducing materials (nitrides, hydrides, sulfides, etc.) or easily oxidized materials may cause fire and explosion hazard
Waste Disposal
Incineration; incinerator equipped with a scrubber or thermal unit to reduce nitrogen oxides emissions.
Properties of Dicyclohexylammonium nitrite
| Melting point: | 182-183 °C (dec.) (lit.) |
| Boiling point: | 370.15°C (rough estimate) |
| Density | 1.0136 (rough estimate) |
| vapor pressure | 3.3 mm Hg ( 25 °C) |
| refractive index | 1.4560 (estimate) |
| storage temp. | 2-8°C |
| Water Solubility | Soluble in water |
| form | powder to crystal |
| color | White to Almost white |
| BRN | 3711911 |
| CAS DataBase Reference | 3129-91-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Dicyclohexyl ammonium nitrite(3129-91-7) |
| EPA Substance Registry System | Dicyclohexylamine nitrite (3129-91-7) |
Safety information for Dicyclohexylammonium nitrite
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Dicyclohexylammonium nitrite
Dicyclohexylammonium nitrite manufacturer
Garuda Chemicals
Earthchem Laboratories P Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
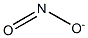


You may like
-
 3129-91-7 Dicyclohexylamine nitrite 98%View Details
3129-91-7 Dicyclohexylamine nitrite 98%View Details
3129-91-7 -
 3129-91-7 98%View Details
3129-91-7 98%View Details
3129-91-7 -
 Dicyclohexylamine Nitrite CAS 3129-91-7View Details
Dicyclohexylamine Nitrite CAS 3129-91-7View Details
3129-91-7 -
 Dicyclohexylamine nitrite CAS 3129-91-7View Details
Dicyclohexylamine nitrite CAS 3129-91-7View Details
3129-91-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1