Dexchlorpheniramine Maleate
Synonym(s):(S)-γ-(4-Chlorophenyl)-N,N-dimethyl-2-pyridinepropanamine maleate salt;S-(+)-Chlorpheniramine maleate salt;Brompheniramine Maleate Impurity B (Ph. Eur.)
- CAS NO.:2438-32-6
- Empirical Formula: C20H23ClN2O4
- Molecular Weight: 390.86
- MDL number: MFCD00079046
- EINECS: 219-450-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
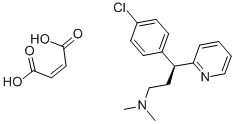
What is Dexchlorpheniramine Maleate?
Absorption
Oral bioavailability in rats 40.5%
Toxicity
Central nervous system depression
Description
Dexchlorpheniramine Maleate is the maleate salt form of dexchlorpheniramine, an alkylamine, and first-generation histamine antagonist with anti-allergic activity. Dexchlorpheniramine maleate competitively blocks H1 receptors, thereby preventing the actions of histamine on bronchial smooth muscle, capillaries and gastrointestinal (GI) smooth muscle. This prevents histamine-induced bronchoconstriction, vasodilation, increased capillary permeability, and GI smooth muscle spasms.
Chemical properties
Dexchlorpheniramine maleate is a white, odourless, crystalline powder which in aqueous solution has a pH of between 4 and 5. It is freely soluble in water, soluble in alcohol and in chloroform, but only slightly soluble in benzene or ether.
Originator
Polaramine,Schering,US,1958
The Uses of Dexchlorpheniramine Maleate
The S-enantiomer of Chlorpheniramine (C424300). Antihistaminic.
The Uses of Dexchlorpheniramine Maleate
Dexchlorpheniramine is an antihistamine used to relieve symptoms of allergy, hay fever, and the common cold. Polaramine (dexchlorpheniramine maleate) is the dextro-isomer of chlorpheniramine maleate and the S-enantiomer of Chlorpheniramine. It is an antihistamine with anticholinergic properties. S-(+)-Chlorpheniramine maleate salt has been used to study the anticholinergic effect of Achillea millefolium and Portulaca olerace on muscarinic receptors of guinea pig tracheal smooth muscle.
Indications
Dexchlorpheniramine can be used in the treatment of perennial and seasonal allergic rhinitis, vasomotor rhiniti, allergic conjunctivitis due to inhalant allergens and foods, mild uncomplicated allergic skin manifestations of urticaria and angioedema, amelioration of allergic reactions to blood or plasma, and dermographism.
Background
Dexchlorpheniramine is the S-enantiomer of chlorpheniramine which is a 1st generation anti-histamine. Dexchlorpheniramine has more pharmacological activity than the R and so is more potent than the racemic mixture.
Definition
ChEBI: Dexchlorpheniramine maleate is an organic molecular entity.
Manufacturing Process
Twenty grams of d-phenylsuccinic acid and 28 grams of 3-(2-pyridyl)-3-pchlorophenyl-N,N-dimethylpropylamine are dissolved in 400 ml of absolute
ethyl alcohol and allowed to stand at room temperature until crystallization is
effected. The crystals are filtered, washed with absolute ethyl alcohol and
recrystallized from 300 ml of this solvent in the same manner. The crystals
are recrystallized twice from 80% ethyl alcohol using 3.5 ml per gram of
compound in the manner described above and pure d-3-(2-pyridyl)-3-pchlorophenyl-N,Ndimethylpropylamine-d-phenylsuccinate is obtained, melting
point 145-147°C.
This salt is shaken with 100 ml of diethyl ether and 50 ml of 20% aqueous
potassium carbonate; the ether layer is separated, dried over anhydrous
potassium carbonate, filtered and the ether is removed in vacuo. The d-3-(2-
pyridyl)-3-p-chlorophenyl-N,N-dimethylpropylamine so obtained is a mobile
oil.
4.3 grams of the above base and 1.8 grams of maleic acid are dissolved in 20
ml isopropyl acetate and kept at room temperature until crystallization is
complete. The crystals are filtered, washed with ethyl acetate and
recrystallized from 15 ml of this solvent in the same manner. The crystalline
d-3-(2-pyridyl)-3-p-chlorophenyl-N,N-dimethylpropylamine maleate so formed
is then filtered off and dried. MP 113-115°C from US Patent 3,030,371.
brand name
Mylaramine (Morton Grove); Polaramine (Schering).
Therapeutic Function
Antihistaminic
General Description
Dexchlorpheniramine(Polaramine) is the dextrorotatory enantiomer of chlorpheniramine.In vitro and in vivo studies of the enantiomers ofchlorpheniramine showed that the antihistaminic activity existspredominantly in the dextro isomer. As mentioned previously,the dextro isomer has the (S) configuration, which issuperimposable on the (S) configuration of the more activelevorotatory enantiomer of carbinoxamine.
Biochem/physiol Actions
H1 histamine receptor antagonist; active isomer.Chlorpheniramine maleate is clinically used as a topical ointment to treat skin disorders such as sunburn, urticaria, angioedema, pruritus and insect bites.
Pharmacokinetics
In allergic reactions, an allergen binds to IgE antibodies on mast cells and basophils. Once this occurs IgE receptors crosslink with each other triggering a series of events that eventually leads to cell-degranulation and the release of histamine (and other chemical mediators) from the mast cell or basophil. Histamine can react with local or widespread tissues through histamine receptors. Histamine, acting on H1-receptors, produces pruritis, vasodilatation, hypotension, flushing, headache, tachycardia, and bronchoconstriction. Histamine also increases vascular permeability and potentiates pain. Dexchlorpheniramine, is a histamine H1 antagonist of the alkylamine class. It competes with histamine for the normal H1-receptor sites on effector cells of the gastrointestinal tract, blood vessels and respiratory tract. It provides effective, temporary relief of sneezing, watery and itchy eyes, and runny nose due to hay fever and other upper respiratory allergies.
Metabolism
Hepatic metabolism. Major metabolism by CYP 2D6 and minor metabolism by 3A4, 2C11 and 2B1.
Mode of action
Dexchlorpheniramine, the d-isomer of the racemic compound chlorpheniramine, is two times more active than chlorpheniramine. Dexchlorpheniramine does not prevent the release of histamine, but rather, competes with free histamine for binding at the H1-receptor sites, and competitively antagonizes the effects of histamine on H1- receptors in the GI tract, uterus, large blood vessels, and bronchial muscle. Blockade of H1-receptors also suppresses the formation of oedema, flare, and pruritus that result from histaminic activity. Since dexchlorpheniramine binds to central and peripheral H1-receptors, sedative effects are likely to occur. H1- antagonists are structurally similar to anticholinergic agents and therefore possess the potential to exhibit anticholinergic properties of varying degrees. They also have antipruritic effects. Dexchlorpheniramine has high antihistaminic activity, moderate anticholinergic effects and minimal sedative effects. The medicine does not possess antiemetic properties.
References
[1] P. E. CHAVES. Assessment of cytotoxicity, genotoxicity, and mutagenicity of the dexchlorpheniramine reference standard and pharmaceutical formula in human peripheral blood mononuclear cells[J]. Brazilian Journal of Pharmaceutical Sciences, 2022, 18 1. DOI:10.1590/s2175-97902022e20096.
[2] GUSTAVO FACCHINI MSC. Ultraviolet A photosensitivity profile of dexchlorpheniramine maleate and promethazine-based creams: Anti-inflammatory, antihistaminic, and skin barrier protection properties[J]. Journal of Cosmetic Dermatology, 2017, 16 4: e59-e67. DOI:10.1111/jocd.12349.
[3] MASAAKI TAGAWA. Neuroimaging of histamine H1-receptor occupancy in human brain by positron emission tomography (PET): a comparative study of ebastine, a second-generation antihistamine, and (+)-chlorpheniramine, a classical antihistamine.[J]. British journal of clinical pharmacology, 2001, 52 5 1: 501-509. DOI:10.1046/J.1365-2125.2001.01471.X.
[4] DONG LIANG. Identification of anthelmintic parbendazole as a therapeutic molecule for HNSCC through connectivity map-based drug repositioning[J]. Acta Pharmaceutica Sinica B, 2022, 12 5: Pages 2429-2442. DOI:10.1016/j.apsb.2021.12.005.
Properties of Dexchlorpheniramine Maleate
| Melting point: | 112-115 °C(lit.) |
| alpha | D25 +44.3° (c = 1 in dimethylformamide) |
| storage temp. | 2-8°C |
| solubility | Very soluble in water, freely soluble in ethanol (96 per cent), in methanol and in methylene chloride. |
| form | neat |
| form | Solid |
| color | White to off-white |
| PH | pH(10g/l, 25℃) : 4.0~5.0 |
| Merck | 13,2198 |
Safety information for Dexchlorpheniramine Maleate
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for Dexchlorpheniramine Maleate
Abamectin manufacturer
Afton Pharma
Supriya Lifescience Ltd
Venkatasai Life Sciences
Amar Healthcare
Nivedita Chemicals Pvt Ltd (Anek Prayog Pvt Ltd)
Mahrshee Laboratories Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 2438-32-6 98%View Details
2438-32-6 98%View Details
2438-32-6 -
 Dexchlorpheniramine maleate 98%View Details
Dexchlorpheniramine maleate 98%View Details
2438-32-6 -
 Dexchlorpheniramine Maleate 99%View Details
Dexchlorpheniramine Maleate 99%View Details -
 Dexchlorpheniramine maleate 2438-32-6 98%View Details
Dexchlorpheniramine maleate 2438-32-6 98%View Details
2438-32-6 -
 DEXCHLORPHENIRAMINE MALEATE 2438-32-6 95-99 %View Details
DEXCHLORPHENIRAMINE MALEATE 2438-32-6 95-99 %View Details
2438-32-6 -
 Dexchlorpheniramine maleate 2438-32-6 98%View Details
Dexchlorpheniramine maleate 2438-32-6 98%View Details
2438-32-6 -
 Dexchlorpheniramine maleate 98% (HPLC) CAS 2438-32-6View Details
Dexchlorpheniramine maleate 98% (HPLC) CAS 2438-32-6View Details
2438-32-6 -
 Dexchlorpheniramine maleate CAS 2438-32-6View Details
Dexchlorpheniramine maleate CAS 2438-32-6View Details
2438-32-6