2-Phenoxyethanol
Synonym(s):PHE;2-Phenoxyethanol;PhG;Ethylene glycol monophenyl ether;DOWANOL EPh
- CAS NO.:122-99-6
- Empirical Formula: C8H10O2
- Molecular Weight: 138.16
- MDL number: MFCD00046644
- EINECS: 204-589-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:42
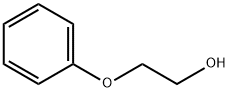
What is 2-Phenoxyethanol?
Toxicity
LC50 oral, rat; 1980 mg/kg . LD50 Rabbit dermal 2250 mg/kg.
2-Phenoxyethanol (PhE) has been shown to induce hepatotoxicity, renal toxicity, and hemolysis at dosages ≥ 400 mg/kg/day in subchronic and chronic studies in multiple species.
The major hazards encountered in the use and handling of 2-phenoxyethanol stem from its toxicologic properties. Toxic by all routes (inhalation, ingestion, and dermal contact), exposure to this very faintly aromatic, colorless, oily liquid may occur from its use.
Description
2-Phenoxyethanol, known early on as ethylene glycol monophenyl ether, is a high-boiling, oily liquid that is moderately water-soluble. It has a long history and several uses in chemical manufacturing and consumer products.
Description
Phenoxyethanol is an organic chemical compound, a glycol ether often used in dermatological products such as skin creams and sunscreen. It is a colorless oily liquid. It is a bactericide (usually used in conjunction with quaternary ammonium compounds). Phenoxyethanol is used in many applications such as cosmetics, vaccines and pharmaceuticals as a preservative.
Chemical properties
clear colorless liquid
Chemical properties
Phenoxyethanol is a colorless, slightly viscous liquid with a faint pleasant odor and burning taste.
Characteristics
Phenoxyethanol is a tried-and-tested preservative, which is welltolerated by the skin and has a low allergy risk. It can be used over a wide pH range. This means that other preservatives can lose their effectiveness if the product is not within the right pH range. It does not smell unpleasant or change the color of the product, which can be the case when using natural antimicrobial substances.
The Uses of 2-Phenoxyethanol
Phenoxyethanol is a preservative used in consumer and health care products, including vaccines, pen inks, ear drops, shampoos, skin cleansers, moisturizers, sun care products, and topical medicaments. The preservative Euxyl-K 400 also contains 2-phenoxyethanol, in combination with methyldibromoglutaronitrile.
The Uses of 2-Phenoxyethanol
Antimicrobial preservative; also used topically in treatment of bacterial infections.
The Uses of 2-Phenoxyethanol
phenoxyethanol is a broad-range preservative with fungicidal, bactericidal, insecticidal, and germicidal properties. It has a relatively low sensitizing factor in leave-on cosmetics. Phenoxyethanol can be used in concentrations of 0.5 to 2.0 percent, and in combination with other preservatives such as sorbic acid or parabens. In addition, it is used as a solvent for aftershaves, face and hair lotions, shampoos, and skin creams of all types. It can be obtained from phenol.
The Uses of 2-Phenoxyethanol
Ethylene glycol phenyl ether at a 1.0% level acts as a preservative in personal care products.
Background
Phenoxyethanol is a colorless liquid with a pleasant odor. It is a glycol ether used as a perfume fixative, insect repellent, antiseptic, solvent, preservative, and also as an anesthetic in fish aquaculture. Phenoxyethanol is an ether alcohol with aromatic properties. It is both naturally found and manufactured synthetically. Demonstrating antimicrobial ability, phenoxyethanol acts as an effective preservative in pharmaceuticals, cosmetics and lubricants.
Production Methods
Phenoxyethanol is prepared by treating phenol with ethylene oxide in an alkaline medium.
Definition
ChEBI: 2-phenoxyethanol is an aromatic ether that is phenol substituted on oxygen by a 2-hydroxyethyl group. It has a role as an antiinfective agent and a central nervous system depressant. It is a primary alcohol, a glycol ether and an aromatic ether. It is functionally related to a phenol.
Synthesis Reference(s)
The Journal of Organic Chemistry, 40, p. 1356, 1975 DOI: 10.1021/jo00897a043
General Description
Colorless liquid with a pleasant odor. Density 1.02 g / cm3. An irritant.
Air & Water Reactions
Oxidizes in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154, 164]. Water soluble.
Reactivity Profile
2-Phenoxyethanol may react violently with strong oxidizing agents. May generate flammable and/or toxic gases with alkali metals, nitrides, and other strong reducing agents. May initiate the polymerization of isocyanates and epoxides.
Health Hazard
May cause moderate eye irritation and moderate corneal injury. Excessive exposure may cause skin irritation and hemolysis.
Fire Hazard
2-Phenoxyethanol is combustible.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Phenoxyethanol is an antimicrobial preservative used in cosmetics
and topical pharmaceutical formulations at a concentration of
0.5–1.0%; it may also be used as a preservative and antimicrobial
agent for vaccines.Therapeutically, a 2.2% solution or 2.0%
cream has been used as a disinfectant for superficial wounds, burns,
and minor infections of the skin and mucous membranes.
Phenoxyethanol has a narrow spectrum of activity and is thus
frequently used in combination with other preservatives,
Industrial uses
2-Phenoxyethanol is used as a preservative in cosmetic formulations at a maximum concentration of 1.0%.
2-Phenoxyethanol is a broad spectrum preservative which has excellent activity against a wide range of Gram negative and Gram positive bacteria, yeast and mould. It is also used as a solvent and, because of its properties as a solvent, it is used in many blends and mixtures with other preservatives.
2-Phenoxyethanol is not registered as a food additive in the EU.
Scognamiglio et al. (ref. 105) reported that 2-phenoxyethanol is a fragrance ingredient used in many fragrance mixtures (see discussion). An ester of 2-Phenoxyethanol, 2-Phenoxyethyl isobutyrate and 2-Phenoxyacetic acid, the main metabolite of 2-Phenoxyethanol, were mentioned in a WHO publication where 43 flavouring agents in food were evaluated (WHO 2003, AR4), however at intakes assessed to be very low in Europe (around 1 µg/kg bw/day).
Contact allergens
Phenoxyethanol is an aromatic ether-alcohol used mainly as a preservative, mostly with methyldibromoglutaronitrile (in Euxyl? K 400) or with parabens. Sensitization to this molecule is very rare.
Pharmacokinetics
This substance has broad-spectrum antimicrobial activity against bacteria, yeasts, and mold .
Side Effects
2-phenoxyethanol is a colorless or straw-colored oily liquid with slight solubility in water but soluble in ethanol and is used as an immersion anesthetic. This agent has bactericidal and fungicidal properties and has been used as a topical anesthetic. This agent can provide deep stages of anesthesia and is relatively inexpensive. Nevertheless, it has no other significant advantages over other drugs. Using 2-phenoxyethanol can elicit several side effects, such as decreased respiratory and heart rate, reduced blood pressure and blood oxygenation, alterations of blood parameters, and elevated plasma levels of adrenaline, glucose, and cortisol, as shown in bighead carp. In addition, sustained and prolonged exposure to 2-phenoxyethanol can cause neuropsychological syndrome in handlers[1].
References
[1] Almut Khler, Karin Finger-Baier, Luis Antunes. "Chapter 16 - Anesthesia, restraint and analgesia in laboratory fishes." Anesthesia and Analgesia in Laboratory Animals (Third Edition) (2023): 393-409.
Safety Profile
Moderately toxic by ingestion and skin contact. A skin and severe eye irritant. Mutation data reported. Some glycol ethers have dangerous human reproductive effects. Combustible when exposed to heat or flame; can react vigorously with oxidizing materials. When heated to decomposition it emits acrid smoke and irritating fumes. To fight fEe, use CO2, dry chemical. Used as a solvent for ester-type resins. See also GLYCOL ETHERS.
Safety
Phenoxyethanol produces a local anesthetic effect on the lips, tongue, and other mucous membranes. The pure material is a moderate irritant to the skin and eyes. In animal studies, a 10% v/v solution was not irritant to rabbit skin and a 2% v/v solution was not irritant to the rabbit eye.Long-term exposure to phenoxyethanol may result in CNS toxic effects similar to other organic solvents.Safety issues related to preservatives used in vaccines, including 2-phenoxyethanol have been reviewed.Contact urticaria has been reported upon exposure to 2-phenoxyethanol-containing cosmetics.
The US FDA has recommended avoiding at least one topical product containing phenoxyethanol due to concerns over inadvertant exposure to nursing infants.
LD50 (rabbit, skin): 5 g/kg
LD50 (rat, oral): 1.26 g/kg
Metabolism
The fate of phenoxyethanol in rats and humans has been investigated . The rate of intestinal absorption was rapid, with 60-70% of the excreted (14)C detected at 3 hours and > 95% of the total 4-day urinary (14)C detected within the first 24 hr. Trace amounts of radioactivity were detected in feces. Four days after dosing, only trace amounts of radioactivity remained in the carcass, primarily in the liver (< 0.2% of the dose), fat and muscle. At the 4 day point, the (14)C concentration in blood was measured to be only 0.001 .
The major metabolite of phenoxyethanol is phenoxyacetic acid .
Storage
Aqueous phenoxyethanol solutions are stable and may be sterilized by autoclaving. The bulk material is also stable and should be stored in a well-closed container in a cool, dry place.
Incompatibilities
The antimicrobial activity of phenoxyethanol may be reduced by interaction with nonionic surfactants and possibly by absorption by polyvinyl chloride.The antimicrobial activity of phenoxyethanol against Pseudomonas aeruginosa may be reduced in the presence of cellulose derivatives (methylcellulose, sodium carboxymethylcellulose, and hypromellose (hydroxypropylmethylcellulose)).
Regulatory Status
Included in the FDA Inactive Ingredients Database (topical
preparations). Included in nonparenteral medicines licensed in the
UK. Included in the Canadian List of Acceptable Non-medicinal
Ingredients.
Under European regulations for cosmetics (76/768/EEC), the
maximum authorized concentration (MAC) of 2-phenoxyethanol is
1.0%.
Properties of 2-Phenoxyethanol
| Melting point: | 11-13 °C (lit.) |
| Boiling point: | 247 °C (lit.) |
| Density | 1.102 g/mL at 25 °C (lit.) |
| vapor density | 4.8 (vs air) |
| vapor pressure | 0.01 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 4620 | 2-PHENOXYETHANOL |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | soluble, clear, colorless to very faintly yellow |
| form | Liquid |
| appearance | colorless, oily liquid |
| pka | 14.36±0.10(Predicted) |
| Specific Gravity | 1.109 (20/4℃) |
| color | Clear colorless |
| Odor | Faint aromatic odor |
| PH | 7 (10g/l, H2O, 23℃) |
| PH Range | 7 at 10 g/l at 23 °C |
| explosive limit | 1.4-9.0%(V) |
| Water Solubility | 30 g/L (20 ºC) |
| Merck | 14,7257 |
| BRN | 1364011 |
| CAS DataBase Reference | 122-99-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Phenoxyethanol(122-99-6) |
| EPA Substance Registry System | Ethylene glycol monophenyl ether (122-99-6) |
Safety information for 2-Phenoxyethanol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 2-Phenoxyethanol
| InChIKey | QCDWFXQBSFUVSP-UHFFFAOYSA-N |
2-Phenoxyethanol manufacturer
Shiv Shakti Trading Corporation
Arcube Science
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 PHENOXYETHANOL 99%View Details
PHENOXYETHANOL 99%View Details -
 Phenoxy Ethanol 99%View Details
Phenoxy Ethanol 99%View Details -
 2 Phenoxyethanol Phenoxy Ethanol, Liquid, Grade: Phenol 5 PpmView Details
2 Phenoxyethanol Phenoxy Ethanol, Liquid, Grade: Phenol 5 PpmView Details
122-99-6 -
 Industrial Grade 2 Phenoxyethanol Liquid, For Preservative, 99.50%View Details
Industrial Grade 2 Phenoxyethanol Liquid, For Preservative, 99.50%View Details
122-99-6 -
 Industrial Grade 2 Phenoxyethanol, 98%, For PreservativeView Details
Industrial Grade 2 Phenoxyethanol, 98%, For PreservativeView Details
122-99-6 -
 PhenoxyethanolView Details
PhenoxyethanolView Details
122-99-6 -
 Industrial Grade Phenoxyethanol 2 Phenoxyethanol, For Preservative, 99.50%View Details
Industrial Grade Phenoxyethanol 2 Phenoxyethanol, For Preservative, 99.50%View Details
122-99-6 -
 2 Phenoxy EthanolView Details
2 Phenoxy EthanolView Details
122-99-6
