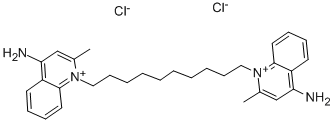
Dequalinium chloride
Synonym(s):1,1′-Decamethylenebis(4-aminoquinaldinium) dichloride;1,1′-Decamethylenebis(4-aminoquinaldinium) dichloride hydrate
- CAS NO.:522-51-0
- Empirical Formula: C30H40Cl2N4
- Molecular Weight: 527.57
- MDL number: MFCD00063502
- EINECS: 208-330-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-16 11:59:48
What is Dequalinium chloride?
Description
Dequalinium Chloride is a a potent and selective non-peptide blocker of the apamin-sensitive small conductance Ca2+-activated K+ channel (IC50 = 1.1 mM).
Chemical properties
White or yellowish-white powder, hygroscopic.
Originator
Dequsan,Sante
The Uses of Dequalinium chloride
Dequalinium Chloride is a quaternary ammonium cation and the active ingredient in various medications including antiseptic and anti-malarial agents.
What are the applications of Application
Dequalinium chloride is a selective blocker of apamin-sensitive K+ channels
Definition
ChEBI: Dequalinium chloride is an organic chloride salt that is the dichloride salt of dequalinium. It has a role as an antiseptic drug, a mitochondrial NADH:ubiquinone reductase inhibitor, an antifungal agent and an antineoplastic agent. It contains a dequalinium.
Manufacturing Process
a) 15 g of 4-aminoquinaldine, 15 g of decamethylene diiodide and 200 ml of
methyl ethyl ketone were refluxed together for 400 hours. The mixture was
allowed to cool, the precipitate filtered off, washed with methyl ethyl ketone,
and 1,1'-decamethylenebis(4-aminoquinaldinium chloride) recrystallized from
ethyl alcohol containing a little methyl alcohol.
b) 160 g of 4-aminoquinaldine, 174 g of decamethylene diiodide and 1,500 ml
of methyl isobutyl carbinol were heated together at 120°C for 90 hours. The
mixture was allowed to cool, the precipitate filtered off, washed with methyl
ethyl ketone and 1,1'-decamethylenebis(4-aminoquinaldinium chloride)
recrystallized from ethyl alcohol containing a little methyl alcohol.
b) 160 g of 4-aminoquinaldine, 174 g of decamethylene diiodide and 1,500 ml
of methyl isobutyl carbinol were heated together at 120°C for 90 hours. The
mixture was allowed to cool, the precipitate filtered off, washed with methyl
ethyl ketone and 1,1'-decamethylenebis(4-aminoquinaldinium chloride)
recrystallized from ethyl alcohol containing a little methyl alcohol.
brand name
Decabis;Dequacaine;Dequafungan;Dequin;Faringina;Gargilon;Grocreme;Labosept;Maltyl;Phylletten;Soor-gel;Sorot;Tetesept.
Therapeutic Function
Antiseptic, Antifungal
World Health Organization (WHO)
Skin reactions to dequalinium chloride, including necrotic lesions, have been reported. It remains available as a mouth and throat disinfectant in many countries.
General Description
Dequalinium Chloride is a topical bacteriostat that is available as various salts. It is used in wound dressings and mouth infections and may also have antifungal action, but may cause skin ulceration.
Biological Activity
Dequalinium is a quaternary ammonium cation with diverse biological activities. It has antifungal activity against C. albicans, C. glabrata, and C. krusei (MICs = 0.5-2, 64-256, and 128 μg/ml, respectively), antitrichomonal activity against T. vaginalis (MICs = 28.8-230.4 μg/ml), and antibacterial activity against a panel of aerobic and facultative anaerobic bacteria (MICs = 0.25-256 μg/ml). Dequalinium inhibits apamin binding to, and net potassium loss mediated by, SKCa channels in guinea pig hepatocytes stimulated by angiotensin II (Item No. 17150). In vivo, dequalinium (1-10 mg/kg) inhibits primary and recurrent tumor growth in a W163 rat colon carcinoma isograft model.
References
1) Miyata?et al.?(2014),?Pharmacologic rescue of an enzyme-trafficking defect in primary hyperoxaluria 1; Proc. Natl. Acad. Sci. USA?111?14406
2) Frey Tirri?et al.?(2011),?Antimicrobial topical agents used in the vagina; Curr. Probl. Dermatol.?40?36
3) Castle?et al.?(1993),?Dequalinium: a potent inhibitor of apamin-sensitive K+ channels in hepatocytes and of nicotinic responses in skeletal muscle; Eur. J. Pharmacol.?236?201
4) Orzaez?et al.?(2011),?Characterization of dequalinium as a XIAP antagonist that targets the BIR2 domain; Apoptosis?16?460
Properties of Dequalinium chloride
| Melting point: | ≥300 °C(lit.) |
| storage temp. | 2-8°C |
| solubility | Slightly soluble in water and in ethanol (96 per cent). |
| form | neat |
| form | Solid |
| color | Beige |
| Merck | 13,2930 |
| Stability: | Stable for 2 years as supplied. Solutions in DMSO or distilled water may be stored at -20° for up to 3 months. |
| CAS DataBase Reference | 522-51-0(CAS DataBase Reference) |
Safety information for Dequalinium chloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dequalinium chloride
| InChIKey | LTNZEXKYNRNOGT-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)
You may like
-
 Dequalinium chloride CAS 522-51-0View Details
Dequalinium chloride CAS 522-51-0View Details
522-51-0 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1