DELAVIRDINE
- CAS NO.:136817-59-9
- Empirical Formula: C22H28N6O3S
- Molecular Weight: 456.56
- MDL number: MFCD00871405
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-24 17:21:45
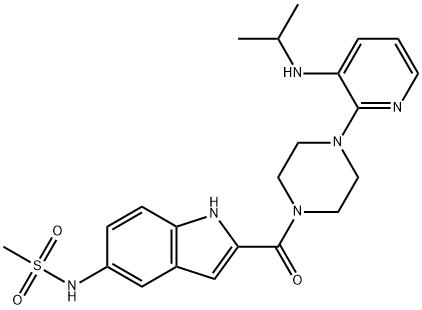
What is DELAVIRDINE?
Absorption
Rapidly absorbed
Toxicity
Major toxicity of delavirdine is rash and should be advised to promptly notify their physician should rash occur. The majority of rashes associated with delavirdine occur within 1 to 3 weeks after initiating treatment with delavirdine. The rash normally resolves in 3 to 14 days and may be treated symptomatically while therapy with delavirdine is continued. Any patient experiencing severe rash or rash accompanied by symptoms such as fever, blistering, oral lesions, conjunctivitis, swelling, muscle or joint aches should discontinue medication and consult a physician.
Description
Delavirdine, a bisheteroarylpiperazine derivative, is a potent nonnucleoside RT inhibitor of activity specific for HIV-1. The U.S. FDA has approved this drug for use in combination with other anti-HIV agents. In Phase I/II study trials, it demonstrated sustained improvements in CD4 cell counts, p24 antigen levels, and RNA viral load. Promising results were obtained when the drug was used in two- or three-drug combinations with nucleoside drugs. Combination of delavirdine with ddI, ddC, or ZDV demonstrated additive or synergistic effects. Delavirdine with ZDV, however, was more beneficial in early HIV infection. Combinations of nevirapine and delavirdine had an antagonistic effect on HIV-1 RT inhibition.
Chemical properties
Crystalline Solid
The Uses of DELAVIRDINE
A bisheteroarylpiperazine (BHAP) reverse transcriptase inhibitor
The Uses of DELAVIRDINE
A bisheteroarylpiperazine (BHAP) reverse transcriptase inhibitor.
Indications
For the treatment of HIV-1 infection in combination with appropriate antiretroviral agents when therapy is warranted
Background
A potent, non-nucleoside reverse transcriptase inhibitor with activity specific for HIV-1.
Definition
ChEBI: The amide resulting from the formal condensation of 5-[(methylsulfonyl)amino]-1H-indole-2-carboxylic acid and 4-amino group of 1-[3-(isopropylamino)pyridin-2-yl]piperazine, delavirdine is a non-nucleoside reverse transcriptase inhibitor ith activity specific for HIV-1. Viral resistance emerges rapidly when delavirdine is used alone, so it is therefore used (as the methanesulfonic acid salt) with other antiretrovirals for combination therapy of HIV infection.
Indications
Delavirdine (Rescriptor) is approved for the treatment of HIV-1 infection in adults and adolescents over age 16 as part of a combination therapy. Rash accompanied by pruritus is the most frequent adverse effect of this agent; however, it usually resolves within several weeks of treatment. Severe skin reactions are rare. Headache, nausea, vomiting, diarrhea, fatigue, and elevated hepatic enzymes also may be associated with delavirdine administration.
brand name
Rescriptor (Agouron).
Acquired resistance
The predominant amino acid substitution associated with resistance is at position 236 of the HIV reverse transcriptase.
General Description
Delavirdine (Rescriptor) must be used with at least twoadditional antiretroviral agents to treat HIV-1 infections.The oral absorption of delavirdine is rapid, and peakplasma concentrations develop in 1 hour. Extensive metabolismoccurs in the liver by CYP isozyme 3A (CYP3A) orpossibly CYP2D6. Bioavailability is 85%. Unlike nevirapine,which is 48% protein bound, delavirdine is more than98% protein bound. The half-life is 2 to 11 hours, andelimination is 44% in feces, 51% in urine, and less than 5%unchanged in urine. Delavirdine induces its own metabolism.Oral dosage forms are supplied as a 200-mg capsuleand a 100-mg tablet.
Pharmaceutical Applications
A complex piperazine derivative, formulated for oral administration.
Mechanism of action
Delavirdine directly inhibits RT and DNA-directed DNA polymerase activities of HIV-1 after the formation of the enzyme–substrate complexes, thereby causing chain-termination effects.
Pharmacokinetics
Delavirdine is a non-nucleoside reverse transcriptase inhibitor (nNRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Delavirdine binds directly to reverse transcriptase (RT) and blocks the RNA-dependent and DNA-dependent DNA polymerase activities by causing a disruption of the enzyme's catalytic site. The activity of Delavirdine does not compete with template or nucleoside triphosphates. HIV-2 RT and eukaryotic DNA polymerases (such as human DNA polymerases alpha, beta, or sigma) are not inhibited by Delavirdine.
Pharmacokinetics
Oral absorption: Not known/available
Cmax 400 mg oral thrice daily: c. 19.3 mg/L
Cmin 400 mg oral thrice daily: c. 8.3 mg/L
Plasma half-life: c. 6 h
Volume of distribution: c. 0.7 L/kg
Plasma protein binding: c. 98%
Absorption and distribution
It is rapidly absorbed following oral administration. Food has no significant effect on absorption. It is distributed predominantly into blood plasma and CNS penetration is poor. The semen:plasma ratio is about 0.02. It is not known if it is distributed into breast milk.
Metabolism and excretion
Several metabolites are formed by the CYP3A4 isoform of cytochrome P450 and it is a potent inhibitor of this enzyme system. Around 44% of the drug is recovered in feces and 51% in urine, about 5% as unchanged drug. Given the predominant hepatic metabolism, caution should be exercised in patients with impaired hepatic function.
Clinical Use
Treatment of HIV disease in adults and children over 12 years of age (in
combination with other antiretroviral agents)
Delavirdine has fallen out of favor with the increasing preference
for antiretrovirals than can be dosed twice or once daily.
Side Effects
Around 18% of patients experience a diffuse, maculopapular, erythematous and often pruritic rash. Dose titration does not appear to reduce the incidence of this side effect. The rash usually first appears within 1 month of commencing therapy and resolves within 2 weeks without dose modification. In about 4% of cases it is severe enough to warrant discontinuation of treatment.
Metabolism
Hepatic
Properties of DELAVIRDINE
| Melting point: | 226-228°C |
| Boiling point: | 732.0±70.0 °C(Predicted) |
| Density | 1.388 |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa1 4.56; pKa2 8.9(at 25℃) |
| color | Pale Yellow |
| Water Solubility | 30g/L(temperature not stated) |
Safety information for DELAVIRDINE
Computed Descriptors for DELAVIRDINE
DELAVIRDINE manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile](https://img.chemicalbook.in/CAS/GIF/500287-72-9.gif)

You may like
-
 Delavirdine 99%View Details
Delavirdine 99%View Details -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1