Pyruvic acid
Synonym(s):α-Ketopropionic acid;2-Oxopropionic acid;Pyruvic acid
- CAS NO.:127-17-3
- Empirical Formula: C3H4O3
- Molecular Weight: 88.06
- MDL number: MFCD00002585
- EINECS: 204-824-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
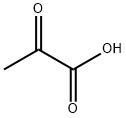
What is Pyruvic acid?
Absorption
Pyruvate is absorbed from the gastrointestinal tract from whence it is transported to the liver via the portal circulation.
Toxicity
Those taking large doses of supplemental pyruvate—usually greater than 5 grams daily—have reported gastrointestinal symptoms, including abdominal discomfort and bloating, gas and diarrhea. One child receiving pyruvate intravenously for restrictive cardiomyopathy died.
Description
Pyruvic acid, the simplest α-ketoacid, plays a central role in sugar metabolism. It is the product of glycolysis, the anaerobic decomposition of glucose. In muscle, it is reduced to lactic acid during exertion. Pyruvic acid was featured in “Emergence” on PBS’s Nova program in 2007.
Chemical properties
Colorless to light yellow liquid
Chemical properties
Pyruvic acid has a sour, acetic odor (similar to acetic acid). It has a pleasant, sour taste with a burning, somewhat sweet note. It tends to darken and decompose unless kept free of minor contaminants and in tightly sealed containers
Occurrence
Isolated from cane sugar fermentation broth and from a few plants; also reported found in peppermint, raw asparagus, leaves and stalk of celery, onion, rutabaga, milk, cream, buttermilk, wheaten bread, blue cheeses, cheddar cheese, cottage cheese, provolone cheese, yogurt, beef, Virginia tobacco, beer, white wine, botrytised wine, cocoa and sake.
The Uses of Pyruvic acid
pyruvic acid is an alpha hydroxy acid that can be irritating and is considered difficult to work with. It has a larger molecular size than the most commonly used AHAs. Sodium pyruvate is more commonly used, and is an organic salt.
The Uses of Pyruvic acid
Pyruvic acid is used as a component in culture broths and media as commercial red seaweed polysaccharide. It is involved in the construction of amino acid alanine as well as supplies energy to living cells via citric acid cycle (Krebs cycle). It reacts with N-acetyl mannosamine by an aldol-type condensation to prepare sialic acid. It is employed to study the cultivation of soil bacteria as micro colonies using soil substrate membrane system. It finds application in liquid chromatography and in the determination of organic acids in red wine.
Indications
For nutritional supplementation, also for treating dietary shortage or imbalance
Background
An intermediate compound in the metabolism of carbohydrates, proteins, and fats. In thiamine deficiency, its oxidation is retarded and it accumulates in the tissues, especially in nervous structures. (From Stedman, 26th ed)
What are the applications of Application
Pyruvic acid is to cultivate undescribed soil bacteria and to develop a new method for determination of organic acids
Definition
Pyruvic acid is an important organic chemical intermediate, an intermediate compound in the metabolism of carbohydrates, proteins, and fats, and is used in a variety of fields, including the pharmaceutical, cosmetic, food, and chemical industries. It has roles as a basic metabolite and cofactor. In thiamine deficiency, its oxidation is retarded and it tends to accumulate in neurostructural tissues causing neurological damage. In vitro studies have shown that pyruvic acid modulates cardiac function at physiological or low doses, but can cause cardiomyocyte damage at high doses.
Preparation
By distillation of tartaric acid in the presence of potassium acid sulfate as a dehydrating agent; from acetyl chloride and potassium cyanide to yield the nitrile, which is subsequently acid hydrolyzed to the acid; pyruvic acid must be rectified under vacuum.
Aroma threshold values
Aroma characteristics at 1.0%: acidic, sweet, caramellic and sour.
Taste threshold values
Taste characteristics at 5 ppm: sharp acidic, sour fruity, with sour creamy and caramellic nuances.
General Description
Pyruvic acid is the key component formed during the hydrolysis of flavor-precursors called S-alk(en)yl-L-cysteine-sulfoxides in onion tissues by allinase during maceration or chopping. The amount of pyruvic acid formed is used as a measure for onion pungency.
Biochem/physiol Actions
Taste at 5 ppm
Biotechnological Applications
Pyruvic acid is a key position in cell metabolism and is involved in many catabolic and anabolic pathways, including glycolysis, gluconeogenesis, amino acid, and protein metabolism. Pyruvic acid is employed for the production of L-tryptophan, L-tyrosine, and 3,4-dihydroxyphenyl alanine in various industries. The diet supplementation with pyruvic acid increased fat loss and minimized the associated loss of body protein. Pyruvic acid is also used in biochemical researches and medicine as a substrate for assaying activities of such enzymes as pyruvate dehydrogenase, pyruvate carboxylase, and pyruvate decarboxylase (Nakazawa et al. 1972; Yamada et al. 1972; Stanko et al. 1992).
Y. lipolytica oxidize glucose and form pyruvic acid (75–80 %) and a-ketoglutaric acid (20–25 %) under thiamine deficiency conditions. The synthesis of the acid was triggered by a decrease in intracellular thiamine concentration to 3.0 lg per 1 g biomass. An approximately 3-fold increase in the amount of the biomass was associated with a subsequent decrease in thiamine content to the level of 1.0 lg per 1 g biomass, whose maximum production of pyruvic acid was 50 g/L in this condition. In addition to glucose, thiamine-auxotrophic yeasts are capable of synthesizing pyruvic acid when grown on glycerol and propionic acid. Technicalgrade glycerol is the most promising raw material for pyruvic acid production. Pyruvic acid was obtained at a concentration of 61 g/L with a yield of 71 % from glycerol (Morgunov et al. 2004; Finogenova et al. 2005).
Pharmacokinetics
Pyruvic acid or pyruvate is a key intermediate in the glycolytic and pyruvate dehydrogenase pathways, which are involved in biological energy production. Pyruvate is widely found in living organisms. It is not an essential nutrient since it can be synthesized in the cells of the body. Certain fruits and vegetables are rich in pyruvate. For example, an average-size red apple contains approximately 450 milligrams. Dark beer and red wine are also rich sources of pyruvate. Recent research suggests that pyruvate in high concentrations may have a role in cardiovascular therapy, as an inotropic agent. Supplements of this dietary substance may also have bariatric and ergogenic applications.
Synthesis
Pyruvic acid's synthesis method is as follows: from Tartaric acid by heating with hydrophilic agents, such as Potassium hydrosulfate.
Metabolism
In the liver, pyruvate is metabolized via several pathways.
Purification Methods
Distil it twice, then fractionally crystallise it by partial freezing. [Beilstein 3 IV 1505.]
Properties of Pyruvic acid
| Melting point: | 11-12 °C (lit.) |
| Boiling point: | 165 °C (lit.) |
| Density | 1.267 g/mL at 25 °C (lit.) |
| vapor pressure | 1.72hPa at 25℃ |
| FEMA | 2970 | PYRUVIC ACID |
| refractive index | n |
| Flash point: | 183 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with chloroform and methanol. |
| form | Liquid |
| pka | 2.39(at 25℃) |
| color | Clear colorless to light yellow or amber |
| PH | 3.11(1 mM solution);2.38(10 mM solution);1.79(100 mM solution); |
| Odor | at 1.00 % in propylene glycol. sharp sour acetic caramellic |
| JECFA Number | 936 |
| Merck | 14,8021 |
| BRN | 506211 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases. Refrigerate. |
| CAS DataBase Reference | 127-17-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Pyruvic acid(127-17-3) |
| EPA Substance Registry System | Propanoic acid, 2-oxo- (127-17-3) |
Safety information for Pyruvic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pyruvic acid
| InChIKey | LCTONWCANYUPML-UHFFFAOYSA-N |
Pyruvic acid manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Pyruvic acid CAS 127-17-3View Details
Pyruvic acid CAS 127-17-3View Details
127-17-3 -
 Pyruvic Acid CAS 127-17-3View Details
Pyruvic Acid CAS 127-17-3View Details
127-17-3 -
 Pyruvic Acid pure CAS 127-17-3View Details
Pyruvic Acid pure CAS 127-17-3View Details
127-17-3 -
 Pyruvic acid CAS 127-17-3View Details
Pyruvic acid CAS 127-17-3View Details
127-17-3 -
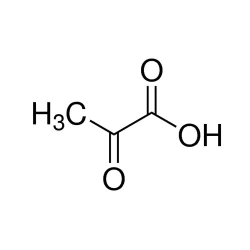 Pyruvic AcidView Details
Pyruvic AcidView Details
127-17-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
