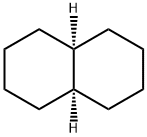
Decahydronaphthalene
Synonym(s):Decahydronaphthalene;Decalin
- CAS NO.:91-17-8
- Empirical Formula: C10H18
- Molecular Weight: 138.25
- MDL number: MFCD00004130
- EINECS: 202-046-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
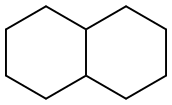
What is Decahydronaphthalene?
Chemical properties
colourless liquid
Physical properties
Clear, colorless, flammable liquid with a mild methanol or hydrocarbon-like odor
The Uses of Decahydronaphthalene
Decahydronaphthalene is widely used as an industrial solvent for resins and fuel additives. It is a substitute for turpentine in lacquers, shoe polishes and waxes.
The Uses of Decahydronaphthalene
Solvent for naphthalene, waxes, fats, oils, resins, rubbers; motor fuel and lubricants; cleaning machinery; substitute for turpentine; shoe-creams; stain remover.
The Uses of Decahydronaphthalene
Solvent for naphthalene, fats, resins, oils; alternate for turpentine in lacquers, shoe polishes, and waxes; component in motor fuels and lubricants
Definition
ChEBI: An ortho-fused bicyclic hydrocarbon that is the decahydro- derivative of naphthalene.
Production Methods
Decalin occurs naturally in crude oil and is produced commercially by the catalytic hydrogenation of naphthalene. It is also a product of combustion and is released from natural fires.
General Description
A clear colorless liquid with an aromatic odor. Flash point 134°F. Less dense than water and insoluble in water. Vapors heavier than air.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
Saturated aliphatic hydrocarbons, such as Decahydronaphthalene, may be incompatible with strong oxidizing agents like nitric acid. Charring of the hydrocarbon may occur followed by ignition of unreacted hydrocarbon and other nearby combustibles. In other settings, aliphatic saturated hydrocarbons are mostly unreactive. They are not affected by aqueous solutions of acids, alkalis, most oxidizing agents, and most reducing agents. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154].
Health Hazard
Inhalation or ingestion irritates nose and throat, causes numbness, headache, vomiting; urine may become blue. Irritates eyes. Liquid de-fats skin and causes cracking and secondary infection; eczema may develop.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Moderately toxic by inhalation and ingestion. Questionable carcinogen with experimental carcinogenic and neoplastigenic data. Mildly toxic by skin contact. Human systemic effects by inhalation: conjunctiva irritation, unspecified olfactory and pulmonary system changes. Can cause kidney damage. Mutation data reported. A skin and eye irritant. Flammable liquid when exposed to heat or flame, can react with oxidzing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes.
Environmental Fate
Photolytic. The following rate constants were reported for the reaction of decahydronaphthalene
and OH radicals in the atmosphere: 1.96 x 10-11 and 2.02 x 10-11 cm3/molecule?sec at 299 K for cis
and trans isomers, respectively (Atkinson, 1985). A photooxidation reaction rate constant of 2.00
x 10-11 was reported for the reaction of decahydronaphthalene (mixed isomers) and OH radicals in
the atmosphere at 298 K (Atkinson, 1990).
Chemical/Physical. Decahydronaphthalene will not hydrolyze because it has no hydrolyzable
functional group.
Purification Methods
Then the organic phase is separated, washed with water, saturated aqueous Na2CO3, again with water, dried with CaSO4 or CaH2 (and perhaps dried further with Na), filtered and distilled under reduced pressure (b 63-70o/10mm). It has also been purified by repeated passage through long columns of silica gel previously activated at 200-250o, followed by distillation from LiAlH4 and storage under N2. Type 4A molecular sieves can be used as a drying agent. Storage over silica gel removes water and other polar substances. [For the separation of cis and trans isomers see Seyer & Walker J Am Chem Soc 60 2125 1938, and Baker & Schuetz J Am Chem Soc 69 1250 1949.]
Properties of Decahydronaphthalene
| Melting point: | −125 °C(lit.) |
| Boiling point: | 189-191 °C(lit.) |
| Density | 0.896 g/mL at 25 °C(lit.) |
| vapor density | 4.76 (vs air) |
| vapor pressure | 42 mm Hg ( 92 °C) |
| refractive index | n |
| Flash point: | 57 °C |
| storage temp. | Store below +30°C. |
| solubility | 0.006g/l (experimental) |
| form | Liquid |
| color | Clear |
| Odor | Aromatic, like turpentine; mild, characteristic. |
| explosive limit | 0.7-4.9%, 100°F |
| Water Solubility | 6 mg/L at 20 ºC |
| Sensitive | Hygroscopic |
| Merck | 14,2846 |
| BRN | 878165 |
| Henry's Law Constant | 7.00, 8.37, 10.6, 11.7, and 19.9 (x 10-2 atm?m3/mol) at 10, 15, 20, 25, and 30 °C, respectively (EPICS, Ashworth et al.,
1988) |
| Dielectric constant | 2.2(20℃) |
| Stability: | Stable. Incompatible with oxidizing agents. Combustible. May form explosive peroxides. Heat and light accelerate peroxide formation. |
| CAS DataBase Reference | 91-17-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Naphthalene, decahydro-(91-17-8) |
| EPA Substance Registry System | Decahydronaphthalene (91-17-8) |
Safety information for Decahydronaphthalene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H304:Aspiration hazard H314:Skin corrosion/irritation H331:Acute toxicity,inhalation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Decahydronaphthalene
| InChIKey | NNBZCPXTIHJBJL-UHFFFAOYSA-N |
Decahydronaphthalene manufacturer
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Decahydronaphthalene 98%View Details
Decahydronaphthalene 98%View Details -
 Decalin (Decahydronaphthalene) pure CAS 91-17-8View Details
Decalin (Decahydronaphthalene) pure CAS 91-17-8View Details
91-17-8 -
 Dekalin, puriss CAS 91-17-8View Details
Dekalin, puriss CAS 91-17-8View Details
91-17-8 -
![Decahydronaphthalene (cis- and trans- mixture) [Testing Methods for Sulfur in Crude Oil and Petroleum Products] CAS 91-17-8](https://img.chemicalbook.in//Content/image/CP5.jpg) Decahydronaphthalene (cis- and trans- mixture) [Testing Methods for Sulfur in Crude Oil and Petroleum Products] CAS 91-17-8View Details
Decahydronaphthalene (cis- and trans- mixture) [Testing Methods for Sulfur in Crude Oil and Petroleum Products] CAS 91-17-8View Details
91-17-8 -
 Dekalin CAS 91-17-8View Details
Dekalin CAS 91-17-8View Details
91-17-8 -
 Decalin (Decahydronaphthalene) 97% CAS 91-17-8View Details
Decalin (Decahydronaphthalene) 97% CAS 91-17-8View Details
91-17-8 -
 DECALIN For Synthesis CAS 91-17-8View Details
DECALIN For Synthesis CAS 91-17-8View Details
91-17-8 -
 Decahydronaphthalene CAS 91-17-8View Details
Decahydronaphthalene CAS 91-17-8View Details
91-17-8
