Dodecane
Synonym(s):n-Dodecane;Dodecane;extraction;n-Dodecane
- CAS NO.:112-40-3
- Empirical Formula: C12H26
- Molecular Weight: 170.33
- MDL number: MFCD00008969
- EINECS: 203-967-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-27 17:16:38
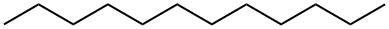
What is Dodecane?
Chemical properties
colourless liquid
Chemical properties
Dodecane, C12H26, is a flammable, colorless liquid with specific gravity 0.749. It occurs in the paraffin fraction of petroleum. Dodecane is released to the environment by wastewater and spills from laboratory and general use of paraffins, petroleum oils, and tars.
Physical properties
Clear, colorless liquid with a mild aliphatic hydrocarbon odor. An odor threshold concentration of 620 ppbv was reported by Nagata and Takeuchi (1990).
The Uses of Dodecane
Dodecane is a component of gasoline and is used as solvent, in organic synthesis, in jet fuel research, as a distillation chaser, and in the rubber and paper processing industries.
The Uses of Dodecane
Solvent; jet fuel research; rubber industry; manufacturing paraffin products; paper processing industry; standardized hydrocarbon; distillation chaser; gasoline component; organic synthesis.
The Uses of Dodecane
n-Dodecane is used as a solvent and a distillation chaser. It finds application as a diluent for tributyl phosphate (TBP) in reprocessing plants and as a possible surrogate for kerosene-based fuels in jet. It is an active component of scintillator as well as used in lubricants and greases.
What are the applications of Application
Dodecane is a nonpolar organic solvent
Definition
ChEBI: A straight-chain alkane with 12 carbon atoms. It has been form the essential oils of various plants including Zingiber officinale (ginger).
Production Methods
Dodecane is isolated from the kerosene and gas oil fractions of crude oil by selective adsorption and subsequent desorption to yield mixtures of paraffins that can be separated by fractional distillation.
Synthesis Reference(s)
The Journal of Organic Chemistry, 44, p. 1014, 1979 DOI: 10.1021/jo01320a033
Tetrahedron Letters, 31, p. 4681, 1990 DOI: 10.1016/S0040-4039(00)97705-0
General Description
Clear colorless liquid.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
Saturated aliphatic hydrocarbons, such as Dodecane, may be incompatible with strong oxidizing agents like nitric acid. Charring of the hydrocarbon may occur followed by ignition of unreacted hydrocarbon and other nearby combustibles. In other settings, aliphatic saturated hydrocarbons are mostly unreactive. They are not affected by aqueous solutions of acids, alkalis, most oxidizing agents, and most reducing agents. When heated sufficiently or when ignited in the presence of air, oxygen or strong oxidizing agents, they burn exothermically to produce carbon dioxide and water.
Fire Hazard
Dodecane is combustible.
Carcinogenicity
Dodecane has been shown to be a promoter of skin carcinogenesis for benzo[a]pyrene and ultraviolet radiation.
Source
Constituent in paraffin fraction of petroleum. Dodecane may be present in stormwater
runoff from asphalted roadways and general use of petroleum oils and tars (quoted, Verschueren).
Schauer et al. (1999) reported dodecane in diesel fuel at a concentration of 15,500 μg/g and in a
diesel-powered medium-duty truck exhaust at an emission rate of 503 μg/kg.
California Phase II reformulated gasoline contained dodecane at a concentration of 136 mg/kg.
Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without catalytic
converters were 83.9 and 1,770 μg/km, respectively (Schauer et al., 2002).
Identified as one of 140 volatile constituents in used soybean oils collected from a processing
plant that fried various beef, chicken, and veal products (Takeoka et al., 1996). Also identified
among 139 volatile compounds identified in cantaloupe (Cucumis melo var. reticulates cv. Sol
Real) using an automated rapid headspace solid phase microextraction method (Beaulieu and
Grimm, 2001).
Environmental Fate
Biological. Dodecane may biodegrade in two ways. The first is the formation of dodecyl
hydroperoxide which decomposes to 1-dodecanol. The alcohol is oxidized forming dodecanoic
acid. The other pathway involves dehydrogenation to 1-dodecene, which may react with water,
giving 1-dodecanol (Dugan, 1972).
Estimated half-lives of dodecane (0.3 μg/L) from an experimental marine mesocosm during the
spring (8–16 °C), summer (20–22 °C), and winter (3–7 °C) were 1.1, 0.7, and 3.6 d, respectively
(Wakeham et al., 1983).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water. Dodecane will
not hydrolyze because it has no hydrolyzable functional group.
Purification Methods
Pass it through a column of Linde type 13X molecular sieves. Store it in contact with, and distil it from sodium. Pass through a column of activated silica gel. It has been crystallised from diethyl ether at -60o. Unsaturated dry material which remained after passage through silica gel has been removed by catalytic hydrogenation (Pt2O) at 45lb/in2 (3.06 atmospheres), followed by fractional distillation under reduced pressure [Zook & Goldey J Am Chem Soc 75 3975 1953]. It has also purified by partial crystallisation from the melt. [Beilstein 1 IV 498.]
Properties of Dodecane
| Melting point: | -9.6 °C (lit.) |
| Boiling point: | 215-217 °C (lit.) |
| Density | 0.75 g/mL at 25 °C (lit.) |
| vapor density | 5.96 (vs air) |
| vapor pressure | 1 mm Hg ( 47.8 °C) |
| refractive index | n |
| Flash point: | 181.4 °F |
| storage temp. | Store below +30°C. |
| solubility | Soluble in acetone, alcohol, chloroform, ether (Weast, 1986), and many hydrocarbons |
| form | Liquid |
| pka | >14 (Schwarzenbach et al., 1993) |
| Specific Gravity | 0.749 (20/4℃) |
| color | Colorless |
| Odor | alkane |
| explosive limit | 0.6%(V) |
| Odor Threshold | 0.11ppm |
| Water Solubility | <0.1 g/100 mL at 25 ºC |
| BRN | 1697175 |
| Henry's Law Constant | 29.7(atm?m3/mol) at 25 °C (calculated from water solubility and vapor pressure, Tolls et al., 2002)
Interfacial tension |
| Dielectric constant | 2.0(20℃) |
| CAS DataBase Reference | 112-40-3(CAS DataBase Reference) |
| NIST Chemistry Reference | n-Dodecane(112-40-3) |
| EPA Substance Registry System | Dodecane (112-40-3) |
Safety information for Dodecane
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H304:Aspiration hazard |
| Precautionary Statement Codes |
P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Dodecane
| InChIKey | SNRUBQQJIBEYMU-UHFFFAOYSA-N |
Dodecane manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran





You may like
-
 112-40-3 1-Dodecane, 99% 98%View Details
112-40-3 1-Dodecane, 99% 98%View Details
112-40-3 -
 n-Dodecane CAS 112-40-3View Details
n-Dodecane CAS 112-40-3View Details
112-40-3 -
 n-Dodecane CAS 112-40-3View Details
n-Dodecane CAS 112-40-3View Details
112-40-3 -
 n-Dodecane CAS 112-40-3View Details
n-Dodecane CAS 112-40-3View Details
112-40-3 -
 Dodecane CAS 112-40-3View Details
Dodecane CAS 112-40-3View Details
112-40-3 -
 Dodecane CAS 112-40-3View Details
Dodecane CAS 112-40-3View Details
112-40-3 -
 Dodecane 98% CAS 112-40-3View Details
Dodecane 98% CAS 112-40-3View Details
112-40-3 -
 Dodecane 99% Anhydrous CAS 112-40-3View Details
Dodecane 99% Anhydrous CAS 112-40-3View Details
112-40-3
