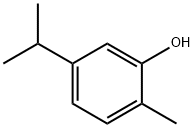
Thymol
Synonym(s):2-Isopropyl-5-methylphenol;5-Methyl-2-(1-methylethyl)phenol;5-Methyl-2-isopropylphenol;IPMP;Thymolum
- CAS NO.:89-83-8
- Empirical Formula: C10H14O
- Molecular Weight: 150.22
- MDL number: MFCD00002309
- EINECS: 201-944-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
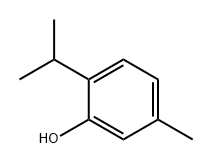
What is Thymol?
Description
Thymol, or 5-methyl-2-isopropyl-phenol, was a component of a thyme plant preparation used by the ancient Egyptians as a mummy preservative. C. Neumann extracted thymol from thyme oil in 1719, and von M. Lallemand synthesized and characterized it in 1842. It is an antimicrobial used in mouthwashes, expectorants, and nail fungus treatments. It is also used to control mites and molds in honeybee colonies. Three new bee-protection products containing thymol are on the market or will be by 2012.
Chemical properties
Thymol occurs as colorless or often large translucent crystals, or as a white crystalline powder with a herbal odor (aromatic and thymelike) and a pungent caustic taste.
Chemical properties
Thymol is the main constituent
of thyme and some origanum oils; it also occurs in many other essential
oils. It forms colorless crystals (mp 51.5°C) with a spicy, herbal, slightly medicinal
odor reminiscent of thyme. Thymol is prepared on a technical scale in a continuous
high-temperature, high-pressure, liquid-phase, ortho-alkylation process,
fromm-cresol and propylene, in the presence of activated aluminumoxide hydrate.
The crude thymol mixture, consisting of approximately 60% thymol, unreacted
m-cresol (about 25%), and other (iso)propyl-substituted products, is separated by
fractional distillation. Most of the by-products are recycled.
Thymol is used as a dry top note in lavender compositions, in men’s fragrances,
and as a disinfectant in oral care products. It is also important as a startingmaterial
for the production of racemic menthol.
Anethole is used in large quantities in the alcoholic beverage industry and
in oral hygiene products. Some crude anethole is converted into anisaldehyde.
Chemical properties
Thymol has a characteristic herbaceous, warm and aromatic odor with a sweet, medicinal, spicy flavor
Occurrence
Reported in the essential oils of Monarda punctata, Satureia thymera, Origanum floribundum, Ocimum viride, Ocimum gratissimum and particularly in thyme (Thymus vulgaris L., T. capitatus, T. serpillum L.), where it is contained up to 50%. Also reported found in citrus peel oils, orange and tangerine juice, bilberry, cranberry, blueberry, papaya, blackberry, celery seed, chive, clove bud, cumin seed, ginger, peppermint oil, corn mint oil, Scotch spearmint oil, nutmeg, parsley, thyme, Gruyere cheese, parmesan cheese, romano cheese, white wine, black tea, plum, sweet and wild marjoram, fenugreek, mango, cardamom, dill herb and seed, licorice, lovage leaf, buckwheat, sweet corn, elder flower, cherimoya, rosemary, lemon balm, Spanish sage, anise hyssop, sweet grass oil, eucalyptus oil and mastic gum oil.
The Uses of Thymol
Thymol is an antimicrobial compound which can increase the efficacy of antibiotics involving drug resistant bacteria. It inhibits growth and lactate production as well as reduces cellular glucose upta ke within the bacteria.
The Uses of Thymol
Thymol is used as a preservative in halothane. It acts as an anesthetic, antiseptic in mouth wash, stabilizer in pharmaceutical preparations. It inhibits the growth and lactate production as well as reduces the uptake of cellular glucose within the bacteria. It is an active ingredient in toothpastes like euthymol. It is involved to control varroa mites and prevent fermentation and the growth of mold in bee colonies.
What are the applications of Application
Thymol is a dye that is also known as 5-Methyl-2-(1-methylethyl)phenol
Background
A phenol obtained from thyme oil or other volatile oils. It is used as a stabilizer in pharmaceutic preparations. It has been used for its antiseptic, antibacterial, and antifungal actions, and was formerly used as a vermifuge. (Dorland, 28th ed)
Production Methods
Thymol is obtained from the volatile oil of thyme (Thymus vulgaris Linne′ (Fam. Labiatae)) by fractional distillation followed by extraction and recrystallization. Thyme oil yields about 20–30% thymol. Thymol may also be produced synthetically from pcymene, menthone, or piperitone, or by the interaction of m-cresol with isopropyl chloride.
Definition
thymol: A pungent-smelling colourless crystalline compound, C10H14O;m.p. 51°C. It occurs in various essentialoils, particularly oil of thyme,and can be made by using iron(III)chloride to oxidize piperitone (itselfextracted from eucalyptus oil). Itsantiseptic properties are exploited ingargles and mouthwashes.
Aroma threshold values
Detection: 86 to 790 ppb
Taste threshold values
Phenolic, medicinal, woody and spicy
General Description
Thymol is a monoterpene phenol derivative. It is a key volatile aroma constituent of the essential oil of plants such as thyme, basil, and oregano. It has strong antioxidant, antibacterial, anticarcinogenesis, anti-inflammatory, and antiseptic properties.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Thymol is a phenolic antiseptic, which has antibacterial and
antifungal activity. However, it is not suitable for use as a
preservative in pharmaceutical formulations because of its low
aqueous solubility. The antimicrobial activity of thymol against
eight oral bacteria has been studied in vitro. Inhibitory activity was
noted against almost all organisms, and a synergistic effect was
observed for combinations of thymol and eugenol, and of thymol
and carvacrol. The activity of thymol against bacteria commonly
involved in upper respiratory tract infections has also been
shown.
Thymol is a more powerful disinfectant than phenol, but its low
water solubility, its irritancy to tissues, and its inactivation by
organic material, such as proteins, limit its use as a disinfectant.
Thymol is chiefly used as a deodorant in antiseptic mouthwashes,
gargles, and toothpastes, such as in Compound Thymol Glycerin
BP, in which it has no antiseptic action.
Thymol is also a true antioxidant and has been used at
concentrations of 0.01% as an antioxidant for halothane,
trichloroethylene, and tetrachloroethylene. The antioxidant activity
of thymol and thymol analogues has been described.
More recently, thymol has been shown to enhance the in vitro
percutaneous absorption of a number of drugs, including 5-
fluorouracil, piroxicam, propranolol, naproxen, and
tamoxifen. Studies have also demonstrated that the melting point
of lidocaine is significantly lowered when it is mixed with
thymol.
The inhalation of thymol, in combination with other volatile
substances, is used to alleviate the symptoms of colds, coughs, and
associated respiratory disorders. Externally, thymol has been used
in dusting powders for the treatment of fungal skin infections;
thymol has been shown to have synergistic antifungal effects when
combined with ketoconazole. Thymol was formerly used in the treatment of hookworm infections but has now been superseded by
less toxic substances.
In dentistry, thymol has been mixed with phenol and camphor to
prepare cavities before filling, and mixed with zinc oxide to form a
protective cap for dentine.
Thymol has been included in food, perfume, and cosmetic
products, and has also been used as a pesticide and fungicide.
Biochem/physiol Actions
Taste at 5 ppm
Clinical Use
Isopropyl m-cresol is extracted from oil of Thymus vulgaris(thyme, of the mint family) by partitioning into alkalineaqueous medium followed by acidification. The crystals obtainedfrom the mother liquor are large and colorless, with athymelike odor. Thymol is only slightly soluble in water,but it is extremely soluble in alcohols and other organic solvents.Thymol has mild fungicidal properties and is used inalcohol solutions and in dusting powders for the treatmentof tinea (ringworm) infections.
Safety Profile
Poison by ingestion and intravenous routes. Moderately toxic by subcutaneous route. Experimental reproductive effects. Mutation data reported. An allergen. Incompatible with acetanilide. When heated to decomposition it emits acrid smoke and irritating fumes. An FDA over-the-counter drug used as an antibacterial and antifungal agent.
Safety
Thymol is used in cosmetics, foods, and pharmaceutical applications
as an excipient. However, thymol may be irritating when
inhaled or following contact with the skin or eyes. It may also cause
abdominal pain and vomiting, and sometimes stimulation followed
by depression of the central nervous system following oral
consumption; fats and alcohol increase absorption and aggravate
symptoms.
Respiratory arrest, attributed to acute nasal congestion and
edema, has been reported in a 3-week-old patient due to the
erroneous intranasal application of Karvol, a combination product
that includes thymol. The patient recovered, but it was recommended
that inhalation decongestants should not be used in
children under the age of 5 years.
LD50 (guinea pig, oral): 0.88 g/kg
LD50 (mouse, IP): 0.11 g/kg
LD50 (mouse, IV): 0.1 g/kg
LD50 (mouse, oral): 0.64 g/kg
LD50 (mouse, SC): 0.243 g/kg
LD50 (rat, oral): 0.98 g/kg
Metabolism
Storage
Thymol should be stored in well-closed, light-resistant containers, in a cool, dry, place. Thymol is affected by light.
Incompatibilities
Thymol is incompatible with iodine, alkalis, and oxidizing agents. It liquefies, or forms soft masses, on trituration with acetanilide, antipyrine, camphor, monobromated camphor, chloral hydrate, menthol, phenol, or quinine sulfate. The antimicrobial activity of thymol is reduced in the presence of proteins.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (inhalation, liquid; oral, powder for solution). Included in nonparenteral medicines (topical creams and ointments) licensed in the UK. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
Properties of Thymol
| Melting point: | 48-51 °C(lit.) |
| Boiling point: | 232 °C(lit.) |
| Density | 0.965 g/mL at 25 °C(lit.) |
| vapor pressure | 1 mm Hg ( 64 °C) |
| refractive index | nD20 1.5227; nD25 1.5204 |
| FEMA | 3066 | THYMOL |
| Flash point: | 216 °F |
| storage temp. | 2-8°C |
| solubility | ethanol: soluble50mg/mL |
| form | Crystals or Crystalline Powder |
| pka | 10.59±0.10(Predicted) |
| color | White |
| Odor | Thyme-like odor |
| PH Range | 7 |
| Water Solubility | 0.1 g/100 mL (20 ºC) |
| Merck | 14,9399 |
| JECFA Number | 709 |
| BRN | 1907135 |
| Stability: | Stable. Incompatible with strong oxidizing agents, organic materials, strong bases. |
| CAS DataBase Reference | 89-83-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 5-methyl-2-(1-methylethyl)-(89-83-8) |
| EPA Substance Registry System | Thymol (89-83-8) |
Safety information for Thymol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Thymol
Thymol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Thymol Crystal 98%View Details
Thymol Crystal 98%View Details -
 Thymol CAS 89-83-8View Details
Thymol CAS 89-83-8View Details
89-83-8 -
 Thymol Crystal(89-83-8)View Details
Thymol Crystal(89-83-8)View Details
89-83-8 -
 Thymol CrystalView Details
Thymol CrystalView Details
89-83-8 -
 THYMOLView Details
THYMOLView Details
89-83-8 -
 Thymol Crystals vampoovuu, Industrial GradeView Details
Thymol Crystals vampoovuu, Industrial GradeView Details
89-83-8 -
 Thymol CrystalsView Details
Thymol CrystalsView Details
89-83-8 -
 White Thymol CrystalsView Details
White Thymol CrystalsView Details
89-83-8