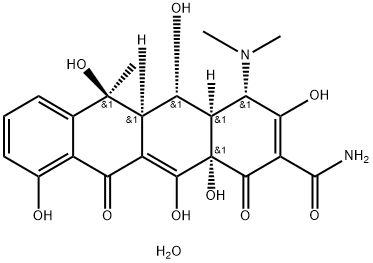
Daunorubicin
- CAS NO.:20830-81-3
- Empirical Formula: C27H29NO10
- Molecular Weight: 527.52
- MDL number: MFCD00866340
- EINECS: 244-069-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
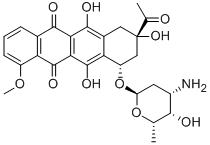
What is Daunorubicin?
Absorption
Daunorubicin was found to have a tmax of 2 h and a cmax of 24.8 μg/mL after a 90 min infusion of the liposomal formulation at a dose of 44 mg/m2.
Description
Daunomycin is a thin red, needle-shaped material.Molecular weight = 527.6; Freezing/Meltingpoint = 190℃ (decomposes). Soluble in water.
Chemical properties
Daunomycin is a thin red, needle-shaped material.
Originator
Cerubidine,Specia,France,1968
The Uses of Daunorubicin
Daunorubicin hydrochloride USP (Cerubidine) is used to traet acute lymphocytic and granulocytic leukemia; lymphomas.
The Uses of Daunorubicin
antineoplastic
The Uses of Daunorubicin
Oncology
Indications
For remission induction in acute nonlymphocytic leukemia (myelogenous, monocytic, erythroid) of adults and for remission induction in acute lymphocytic leukemia of children and adults.
Daunorubicin is indicated in combination with cytarabine for the treatment of newly-diagnosed therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC) in adults and pediatric patients 1 year and older.
Background
A very toxic anthracycline aminoglycoside antineoplastic isolated from Streptomyces peucetius and others, used in treatment of leukemia and other neoplasms.
Definition
ChEBI: A natural product found in Actinomadura roseola.
Indications
Daunorubicin (Cerubidine) is used to treat acute leukemias, while its structural analogue, doxorubicin (Adriamycin) is extensively employed against a broad spectrum of cancers. Although the two drugs have similar pharmacological and toxicological properties, doxorubicin is more potent against most animal and human tumors and will be discussed in greater detail.
Manufacturing Process
Two 300 ml Erlenmeyer flasks are prepared, each of them containing 60 ml of
the following vegetative medium in tap water: 0.6% peptone, 0.3% dry yeast
and 0.05% calcium nitrate. The pH after sterilization by heating in an
autoclave to 120°C for 20 minutes is 7.2.
Each flask was inoculated with mycelium of Streptomyces F.I. 1762 whose
quantity corresponds to one-fifth of a suspension in sterile water of the
mycelium of a 10 day old culture growth in a test tube containing the
following ingredients dissolved in tap water.
Percent
Saccharose
2
Dry yeast
0.1
Potassium hydrogen phosphate
0.2
Percent
Sodium nitrate
0.2
Magnesium sulfate
0.2
Agar
2
The flasks are incubated at 28°C for 48 hours on a rotary shaker with a stroke
of 60 mm at 220 rpm. 2 ml of a vegetative medium thus grown are used to
inoculate 300 ml Erlenmeyer flasks containing 60 ml of the following
productive medium in tap water at pH 7.0.
Percent
Glucose 4
Dry yeast 1.5
Sodium chloride 0.2
Potassium hydrogen phosphate 0.1
Calcium carbonate 0.1
Calcium carbonate 0.1
Iron sulfate 0.001
Zinc sulfate 0.001
Copper sulfate 0.001
(The medium had been sterilized at 120°C for 20 minutes, the glucose being
previously sterilized separately at 110°C for 20 minutes.) It is incubated at
28°C under the conditions described for the vegetative media. After 120 hours
of fermentation a maximum activity corresponding to a concentration of 60
micrograms/ml is achieved.
brand name
Cerubidine (Bedford); Cerubidine (Sanofi Aventis); Cerubidine (Wyeth).
Therapeutic Function
Cancer chemotherapy
General Description
Anthracycline antibiotic. An anticancer agent.
Biological Activity
daunorubicin is an inhibitor of dna topoisomerase ii [1].daunorubicin is an anthracycline antibiotic. it is also used as an effective chemotherapeutic agent against tumors especially acute myeloid leukaemia and acute lymphocytic leukaemia. daunorubicin can affect the metabolism and synthesis of dna and rna. in the in vitro assay, daunorubicin inhibits the incorporation of thymidine and uridine into l1210 cells. it also inhibits the incorporation of labeled precursors into the isolated dna and rna from incubated cells. when treated with leukemic cells isolated from acute lymphocytic leukemia patients, daunorubicin significantly inhibits the biosynthesis of the dna and rna macromolecules [2, 3].
Pharmacokinetics
Daunorubicin is an anthracycline antibiotic and antineoplastic agent. It acts by inhibiting cellular reproduction through interference with DNA replication although it may contribute to the induction of cell death by increasing oxidative stress through the generation of reactive oxygen species and free radicals. As an antineoplastic agent, daunorubicin carries significant toxicities including cytopenias, hepatotoxicity, and extravasation reactions. Like other anthracyclines, daunorubicin also exhibits cardiotoxicity in proportion with the cumulative dose received over time.
Clinical Use
Antineoplastic agent:
Acute leukaemias
HIV-related Kaposi’s Sarcoma
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Human poison by ingestion. Experimental poison by subcutaneous, intravenous, and intraperitoneal routes. Experimental teratogenic and reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx. See also DAUNOMYCIN HYDROCHLORIDE.
Potential Exposure
An antibiotic. It is used as a medicine for treating cancer.
Drug interactions
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine due to risk of
agranulocytosis.
Cytotoxics: possible increased cardiotoxicity with
trastuzumab - avoid for up to 28 weeks after
stopping trastuzumab.
Avoid with live vaccines.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Metabolism
Metabolism
Daunorubicin is rapidly taken up by the tissues, especially
by the kidneys, liver, spleen and heart. Subsequent release
of drug and metabolites is slow (half-life ~55 hours). It is
rapidly metabolised in the liver and the major metabolite,
daunorubicinol is also active.
It is excreted slowly in the urine, mainly as metabolites
with 25% excreted within 5 days. Biliary excretion
accounts for 40-50% elimination
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with daunomycin you should be trained on its proper handling and storage. A regulated, marked area should be established wherethis chemical is handled, used, or stored in compliance withOSHA Standard 1910.1045. Store in tightly closed containers in a cool, well-ventilated area.
Shipping
UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1- Poisonous materials, Technical Name Required.
Waste Disposal
It is inappropriate and possi- bly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quanti- ties of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceuti- cal shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste con- taining this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
References
[1] hande k r. etoposide: four decades of development of a topoisomerase ii inhibitor. european journal of cancer, 1998, 34(10): 1514-1521.
[2] momparler r l, karon m, siegel s e, et al. effect of adriamycin on dna, rna, and protein synthesis in cell-free systems and intact cells. cancer research, 1976, 36(8): 2891-2895.
[3] meriwether w d, bachur n r. inhibition of dna and rna metabolism by daunorubicin and adriamycin in l1210 mouse leukemia. cancer research, 1972, 32(6): 1137-1142.
Properties of Daunorubicin
| Melting point: | 155 °C |
| Boiling point: | 608.13°C (rough estimate) |
| Density | 1.3522 (rough estimate) |
| refractive index | 1.6400 (estimate) |
| storage temp. | Store at -20°C, protect from light |
| solubility | ≥83.33 mg/mL in DMSO |
| form | Powder |
| pka | pKa 4.92±0.16(H2O t=25±0.5 I=0.03) (Uncertain) |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 20830-81-3 |
| IARC | 2B (Vol. 10, Sup 7) 1987 |
| EPA Substance Registry System | Daunomycin (20830-81-3) |
Safety information for Daunorubicin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P330:Rinse mouth. P362:Take off contaminated clothing and wash before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Daunorubicin
Daunorubicin manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1