Azithromycin
Synonym(s):N-Methyl-11-aza-10-deoxo-10-dihydroerythromycin A;Azithromycin;Azithromycin dihydrate;CP 62,993;CP-62993
- CAS NO.:83905-01-5
- Empirical Formula: C38H72N2O12
- Molecular Weight: 748.98
- MDL number: MFCD00873574
- EINECS: 617-500-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
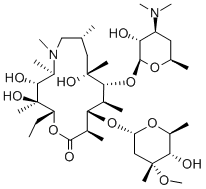
What is Azithromycin?
Absorption
Bioavailability of azithromycin is 37% following oral administration. Absorption is not affected by food. Macrolide absorption in the intestines is believed to be mediated by P-glycoprotein (ABCB1) efflux transporters, which are known to be encoded by the ABCB1 gene .
Toxicity
Possible major adverse effects include cardiovascular arrhythmias and hearing loss. Macrolide resistance is also an ongoing issue. Hepatotoxicity has been observed in rare cases.
Due to the act that azithromycin is mainly eliminated by the liver, caution should be observed when azithromycin is given to patients with decreased hepatic function.
Because limited data in patients with renal GFR<10 mL/min, caution should be exercised when prescribing azithromycin to these patients.
Use in Pregnancy: Azithromycin is categorized as a pregnancy category B drug. It should be used during pregnancy only if clearly needed.
Nursing Mothers: Because many other drugs are excreted in human milk, caution should be observed when azithromycin is given to a nursing woman.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term studies in animals have not been performed to study carcinogenic potential. Azithromycin has demonstrated no potential to be mutagenic in standard laboratory tests. No evidence of negative effects on fertility due to azithromycin was found.
Description
Azithromycin was the first of the azalides and was designed to improve the stability and biological half-life of erythromycin A, as well as improve activity against Gram negative bacteria. Azithromycin is a long-acting macrolide antibiotic structurally related to erythromycin A (EA), having a methyl-substituted nitrogen at position 9a in the aglycone ring.
Chemical properties
White Crystalline Powder
Originator
Pliva (Yugoslavia)
The Uses of Azithromycin
Azithromycin is an azalide macrolide antibiotic.
Background
Azithromycin is a broad-spectrum macrolide antibiotic with a long half-life and a high degree of tissue penetration . It was initially approved by the FDA in 1991.
It is primarily used for the treatment of respiratory, enteric and genitourinary infections and may be used instead of other macrolides for some sexually transmitted and enteric infections.
In March 2020, a small study was funded by the French government to investigate the treatment of COVID-19 with a combination of azithromycin and the anti-malaria drug hydroxychloroquine. The results were positive, all patients taking the combination were virologically cured within 6 days of treatment, however, larger studies are required.
Indications
Azithromycin is indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the microorganisms listed in the specific conditions below. Recommended dosages, duration of therapy and considerations for various patient populations may vary among these infections.
Adults:
Acute bacterial exacerbations of chronic obstructive pulmonary disease due to Haemophilus influenzae, Moraxella catarrhalis or Streptococcus pneumoniae
Acute bacterial sinusitis due to Haemophilus influenzae, Moraxella catarrhalis or Streptococcus pneumoniae
Community-acquired pneumonia due to Chlamydophila pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae or Streptococcus pneumoniae in patients appropriate for oral therapy
Pharyngitis/tonsillitis caused by Streptococcus pyogenes as an alternative to first-line therapy in individuals who cannot use first-line therapy. Uncomplicated skin and skin structure infections due to Staphylococcus aureus, Streptococcus pyogenes, or Streptococcus agalactiae. Abscesses usually require surgical drainage.
Urethritis and cervicitis due to Chlamydia trachomatis or Neisseria gonorrhoeae.
Genital ulcer disease in men due to Haemophilus ducreyi (chancroid). Due to the small number of women included in clinical trials, the efficacy of azithromycin in the treatment of chancroid in women has not been established.
Indications
Azithromycin in a 500-mg dose three times a week has been shown to yield a 60% reduction in inflammatory papules in 83% of patients enrolled in a 12- week study. There is no associated pseudotumor cerebri and, therefore, it can be used for an acne flare during early Accutane therapy.
Definition
ChEBI: Azithromycin is a macrolide antibiotic useful for the treatment of bacterial infections. It is an azalide, derived from erythromycin, and a member of a subclass of macrolide antibiotics with bacteriocidal and bacteriostatic activities.
Manufacturing Process
To a solution of 0.54 g (0.000722 mole) of 11 aza-10-deoxo-10- dihydroerythromycin A in 20 ml CHCl3 were added 0.0589 ml (0.000741 mole) of formaldehyde (approx. 35% w./w.) and 0.00283 g (0.000735 mole) of formic acid (approx. 98 to 100% w./w.). The reaction mixture was stirred for 8 hours while heating under reflux, then cooled to ambient temperature, whereupon were added 15 ml of water (pH 5.8). The pH of the reaction mixture was adjusted to 5.0 by means of 2 N HCl, whereupon the CHCl3 layer was separated. To the aqueous part was added 15 ml of CHCl3, the pH of the reaction suspension was adjusted to 7.5 by means of 20% NaOH, the layers were separated and subsequently the aqueous layer was extracted three times with 15 ml of CHCl3. The combined chloroform extracts having pH 7.5 were dried over K2CO3 and evaporated under reduced pressure, yielding 0.45 g (82.4%) of N-methyl-11-aza-10-deoxo-10-dihydro erythromycin A (azithromycin), m.p. 113°-115°C. [α]D 20= - 37.0 (1% in CHCl3).
brand name
Zithromax (Pfizer); Zmax (Pfizer);Sunamed.
Therapeutic Function
Antibiotic
Antimicrobial activity
It is less potent than erythromycin A against Gram-positive isolates, but is more active against Gram-negative bacteria. It is four times more potent than erythromycin A against H. influenzae, N. gonorrhoeae and Campylobacter spp., and twice as active against Mor. catarrhalis. It also exhibits superior potency against Enterobacteriaceae, notably Esch. coli, Salmonella enterica serotypes, and Shigella spp. It is active against Mycobacteria, notably the M. avium complex and against intracellular micro-organisms such as Legionella and Chlamydia spp.
Chemical modification at the 9 position of the erythronolide A ring of erythromycin A blocks the internal ketalization and markedly improves acid stability. At pH 2, loss of 10% activity occurred in less than 4 s with erythromycin A, but took 20 min with azithromycin. The AUC at 0–24 h is 4.5 mg.h/L. The level is only slightly increased on repeated dosing.
Binding to plasma protein varies with the concentration, from around 50% at 0.05 mg/L to 7.1% at 1 mg/L. The apparent elimination half-life is dependent upon sampling interval: between 8 and 24 h it ranged from 11 to 14 h; between 24 and 72 h it was 35–40 h.
It rapidly penetrates the tissues, reaching levels that approach or, in some cases, exceed the simultaneous plasma levels and persist for 2–3 days. Only about 6% of the dose is found in urine in the first 24 h.
General Description
The spectrum of antimicrobial activity of azithromycin issimilar to that observed for erythromycin and clarithromycinbut with some interesting differences. In general,it is more active against Gram-negative bacteria and less activeagainst Gram-positive bacteria than its close relatives.The greater activity of azithromycin against H. influenzae,M. catarrhalis, and M. pneumoniae coupled with its extendedhalf-life permits a 5-day dosing schedule for thetreatment of respiratory tract infections caused by thesepathogens. The clinical efficacy of azithromycin in the treatmentof urogenital and other sexually transmitted infectionscaused by Chlamydia trachomatis, N. gonorrhoeae, H.ducreyi, and Ureaplasma urealyticum suggests that singledosetherapy with it for uncomplicated urethritis or cervicitismay have advantages over use of other antibiotics.
Biological Activity
Azithromycin is a macrolide antibiotic. It is active against S. pneumoniae, S. aureus, N. gonorrhoeae, M. pneumoniae, H. pylori, C. trachomatis, and H. influenzae in vitro (MIC90s = <0.01-2 mg/L). Azithromycin increases survival in mouse models of intraperitoneal S. pyogenes, S. pneumoniae, E. faecalis, or H. influenzae infection (ED50s = 0.78, 8.7, 12.7, and 30.3 mg/kg, respectively). It inhibits replication of severe acute respiratory coronavirus 2 (SARS-CoV-2), but not Middle East respiratory syndrome CoV (MERS-CoV), when used at concentrations of 5 and 10 μM. Azithromycin also decreases plasma levels of IL-6, TNF-α, and IL-1β and increases survival in a mouse model of LPS-induced sepsis when administered at a dose of 100 mg/kg. Formulations containing azithromycin have been used in the treatment of a variety of bacterial infections.
Pharmacokinetics
Macrolides stop bacterial growth by inhibiting protein synthesis and translation, treating bacterial infections . Azithromycin has additional immunomodulatory effects and has been used in chronic respiratory inflammatory diseases for this purpose .
Pharmacokinetics
Oral absorption:37%
Cmax 250 mg oral: 0.17 mg/L after 2.2 h
500 mg oral: 0.4 mg/L after2h
Plasma half-life (terminal): 11–40 h
Volume of distribution: 31 L/kg
Plasma protein binding :7–50%
Chemical modification at the 9 position of the erythronolide A ring of erythromycin A blocks the internal ketalization and markedly improves acid stability. At pH 2, loss of 10% activity occurred in less than 4 s with erythromycin A, but took 20 min with azithromycin. The AUC at 0–24 h is 4.5 mg.h/L. The level is only slightly increased on repeated dosing.
Binding to plasma protein varies with the concentration, from around 50% at 0.05 mg/L to 7.1% at 1 mg/L. The apparent elimination half-life is dependent upon sampling interval: between 8 and 24 h it ranged from 11 to 14 h: between 24 and 72 h it was 35–40 h.
It rapidly penetrates the tissues, reaching levels that approach or, in some cases, exceed the simultaneous plasma levels and persist for 2–3 days. Only about 6% of the dose is found in urine in the first 24 h.
Clinical Use
This product is suitable for the treatment of the following symptoms of infection caused by susceptible bacteria:
1. For acute pharyngitis and acute tonsillitis caused by Streptococcus pyogenes and for nasosinusitis, acute otitis media, acute bronchitis, and acute exacerbation of chronic bronchitis caused by sensitive bacteria.
2. For pneumonia caused by Mycoplasma pneumonia, Streptococcus pneumonia and Haemophilus influenza.
3. For urethritis, cervicitis and pelvic inflammatory disease caused by chlamydia and non-multiple drug resistance Neisseria gonorrhoeae, and for simple genital infection caused by non-multiple drug resistance Neisseria gonorrhoeae (exclude co-infection of Misu spirochetes).
4. For skin and soft tissue infections caused by sensitive bacteria.
Side Effects
Azithromycin is well tolerated with little gastrointestinal disturbance.
Veterinary Drugs and Treatments
Azithromycin with its relative broad spectrum and favorable pharmacokinetic
profile may be useful for a variety of infections in
veterinary species. Little data is published at this time, however.
Azithromycin has been shown to be ineffective in the treatment of
Mycoplasma haemofelis in cats.
Azithromycin may be potentially useful for treating Rhodococcus
infections in foals.
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: increased toxicity with
disopyramide; increased risk of ventricular
arrhythmias with dronedarone - avoid.
Antibacterials: possibly increased rifabutin
concentration (increased risk of uveitis and
neutropenia) - reduce dose of rifabutin.
Anticoagulants: effect of coumarins may be enhanced.
Antidepressants: the manufacturer of reboxetine
advises to avoid concomitant use.
Antihistamines: may inhibit the metabolism of
mizolastine (risk of hazardous arrhythmias) - avoid.
Antimalarials: avoid with artemether/lumefantrine;
increased risk of ventricular arrhythmias with
piperaquine with artenimol - avoid.
Antipsychotics: increased risk of ventricular
arrhythmias with droperidol - avoid.
Antivirals: concentration possibly increased by ritonavir.
Ciclosporin: may inhibit the metabolism of
ciclosporin (increased ciclosporin levels).
Colchicine: treatment with both agents has been
shown in a study to increase the risk of fatal
colchicine toxicity, especially in patients with renal
impairment - avoid.
Ergot alkaloids: increased risk of ergotism - avoid.
Statins: possible increased risk of myopathy with
atorvastatin and simvastatin.
Structure
Azithromycin is structurally related to erythromycin. Azithromycin [9-deoxo-9a-aza-9a-methyl-9a-homoerythromycin] is a part of the azalide subclass of macrolides, and contains a 15-membered ring, with a methyl-substituted nitrogen instead of a carbonyl group at the 9a position on the aglycone ring, which allows for the prevention of its metabolism. This differentiates azithromycin from other types of macrolides.
Metabolism
In vitro and in vivo studies to assess the metabolism of azithromycin have not been performed , however, this drug is eliminated by the liver , .
Metabolism
In pharmacokinetic studies it has been demonstrated that the concentrations of azithromycin measured in tissues are noticeably higher (as much as 50 times) than those measured in plasma, which indicates that the agent strongly binds to tissues. Azithromycin is excreted in bile mainly as unchanged drug. Ten metabolites have also been detected in bile, which are formed through N- and O- demethylation in the liver, hydroxylation of desosamine - and aglycone rings and cleavage of cladinose conjugate. The metabolites of azithromycin are not microbiologically active
Storage
-20°C
Mode of action
Azithromycin reversibly binds to the 50S ribosomal subunit of the 70S ribosome of sensitive microorganisms, thereby inhibiting the translocation step of protein synthesis, wherein a newly synthesized peptidyl tRNA molecule moves from the acceptor site on the ribosome to the peptidyl (donor) site, and consequently inhibiting RNA-dependent protein synthesis leading to cell growth inhibition and cell death.
Properties of Azithromycin
| Melting point: | 113-115°C |
| Boiling point: | 822.1±65.0 °C(Predicted) |
| alpha | D20 -37° (c = 1 in CHCl3) |
| Density | 1.18±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Practically insoluble in water, freely soluble in anhydrous ethanol and in methylene chloride. |
| form | neat |
| pka | pKa 8.74 (H2O t=25 I=0.167) (Uncertain);9.45(H2O t=25 I=0.167) (Uncertain) |
| form | Solid |
| color | White to Off-White |
| optical activity | [α]/D 45±2°, c = 1% in ethanol |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 83905-01-5(CAS DataBase Reference) |
| EPA Substance Registry System | Azithromycin (83905-01-5) |
Safety information for Azithromycin
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
Computed Descriptors for Azithromycin
Azithromycin manufacturer
The Bangalore Sales Corporation
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
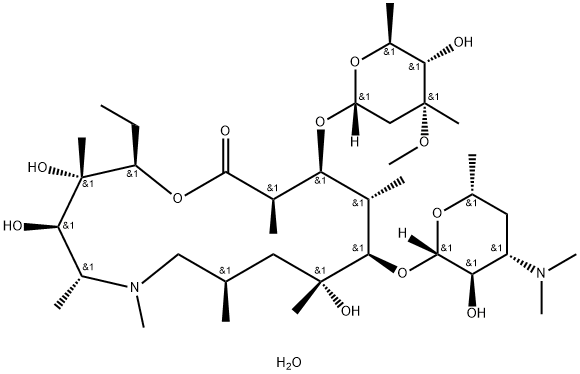
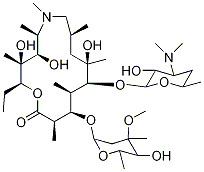






You may like
-
 Azithromycin 98%View Details
Azithromycin 98%View Details -
 AZITHROMYCIN 99%View Details
AZITHROMYCIN 99%View Details -
 Azithromycin 83905-01-05 98%View Details
Azithromycin 83905-01-05 98%View Details
83905-01-05 -
 Azithromycin 99%View Details
Azithromycin 99%View Details -
 Azithromycin 99%View Details
Azithromycin 99%View Details -
 Azithromycin Powder IP, 25KgView Details
Azithromycin Powder IP, 25KgView Details
117772-70-0 -
 Azithromycin API PowderView Details
Azithromycin API PowderView Details
117772-70-0 -
 Azithromycin Powder ......, 25KgView Details
Azithromycin Powder ......, 25KgView Details
117772-70-0
