Cyclohexanol
Synonym(s):Hexahydrophenol
- CAS NO.:108-93-0
- Empirical Formula: C6H12O
- Molecular Weight: 100.16
- MDL number: MFCD00003855
- EINECS: 203-630-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:05
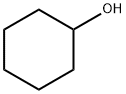
What is Cyclohexanol?
Description
Cyclohexanol is a sticky solid (above 25℃/77°F) or colorless, viscous liquid with a faint camphorodor. Odor threshold=0.07 ppm. Molecularweight=100.16; Specific gravity (H2O:1)=0.96; Boilingpoint=161℃; Freezing/Melting point=24℃; Vaporpressure=mmHg at 25℃; Flash point=68℃;Autoignition temperature=300℃. The explosive limitsare: LEL=2.4%; UEL=12%. Hazard Identification (basedon NFPA-704 M Rating System): Health 1, Flammability 2,Reactivity 0. Slight solubility in water; solubility=4% at20℃.
Chemical properties
Cyclohexanol is a sticky solid (above 25°C /77°C F) or colorless, viscous liquid. Faint camphor odor.It has a fairly high flash point and boiling range. A solvent for cellulose ethers, ester gum,shellac, low viscosity silicones and polyvinyl chloride. lt has a fairly limited use in screen inks. lt is miscible with oils and hydrocarbon solvents.
Physical properties
Clear, colorless to pale yellow, viscous, hygroscopic liquid with a camphor-like odor. A detection odor threshold concentration of 64 μg/m3 (155 ppbv) was reported by Punter (1983).
The Uses of Cyclohexanol
Cyclohexanol is used for the productionof adipic acid and caprolactam for makingnylon. Its phthalate derivatives are used forplasticizers. It is used as a stabilizer for soapsand detergents; as a solvent for lacquers,varnishes, and shellacs; and as a dye solventfor textiles.
The Uses of Cyclohexanol
Solvent for oils, resins, ethyl cellulose; manufacture of soap, plastics
What are the applications of Application
Cyclohexanol is used as a precursor to nylons and plasticizers
Definition
ChEBI: Cyclohexanol is an alcohol that consists of cyclohexane bearing a single hydroxy substituent. The parent of the class of cyclohexanols. It has a role as a solvent. It is a secondary alcohol and a member of cyclohexanols.
Production Methods
Cyclohexanol is prepared by the catalytic air oxidation of cyclohexane or by the catalytic hydrogenation of phenol. The most important use of cyclohexanol is in producing adipic acid used in the manufacture of caprolactam. Cyclohexanol is used in the manufacture of esters for use as plasticizers; it is also used as a chemical intermediate, a stabilizer, a homogenizer for various soap and detergent emulsions, and as a solvent for lacquers and varnishes . The primary routes of occupational exposure are dermal and inhalation.
Synthesis Reference(s)
Journal of the American Chemical Society, 97, p. 7118, 1975 DOI: 10.1021/ja00857a025
Tetrahedron Letters, 21, p. 2305, 1980 DOI: 10.1016/S0040-4039(00)92591-7
General Description
A colorless liquid with a camphor-like odor. Soluble in most organic liquids. Flash point 154°F. May be toxic by inhalation or skin exposure. Vapors are narcotic in high concentrations. Irritates skin, eyes and mucus membranes. Used in making soap, lacquers, and plastics.
Air & Water Reactions
Less dense than water and slightly soluble in water.
Reactivity Profile
Cyclohexanol is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. Violent reaction with nitric acid. Incompatible with strong oxidizers (chromium trioxide, nitric acid, etc.).
Health Hazard
Cyclohexanol is moderately toxic. Targetorgans are the eyes, skin, and respiratorysystem. At high concentrations cyclohexanolabsorbed through the skin may possiblyinjure the brain, kidney, and heart.
LD50 value, single oral dose (rats): 2060mg/kg
LD50 value, single intravenous dose (mice):270 mg/kg
Inhalation of vapors may cause irritationof the eyes, nose, and throat. However,because of its low vapor pressure (1.12 torrat 25°C), the health hazard due to inhalationis low. Ingestion can cause nausea,trembling, and gastrointestinal disturbances.Repeated skin contact may produce erythemaand edema.
Flammability and Explosibility
Not classified
Chemical Reactivity
Reactivity with Water: No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Potential Exposure
Cyclohexanol is used in making plac- ticizers, dry cleaning, dyebath; and textile finishing formu- lations; as a solvent for ethyl cellulose and other resins; it is used in soap manufacture; it is used as a raw material for adipic acid manufacture; as a nylon intermediate.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Solubility in organics
4% soluble in water, miscible with alcohol and oils. Cyclohexanol will dissolve 11% water at room temperature.
Storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with cyclohexanol you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist.Cyclohexanol must be stored to avoid contact with strongoxidizers (such as chlorine, bromine, and fluorine), sinceviolent reactions occur. Metal containers involving thetransfer of this chemical should be grounded and bonded.Where possible, automatically pump liquid from drums orother storage containers to process containers. Drums mustbe equipped with self-closing valves, pressure vacuum bungs,and flame arresters. Use only nonsparking tools and equipment, especially when opening and closing containers of thischemical. Sources of ignition, such as smoking and openflames, are prohibited where this chemical is used, handled,or stored in a manner that could create a potential fire orexplosion hazard. Wherever this chemical is used, handled,manufactured, or stored, use explosion-proof electrical equipment and fittings.
Shipping
UN1986 Alcohols, toxic, flammable, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, 6.1-Poison Inhalation Hazard, Technical Name Required.
Purification Methods
Reflux it with freshly ignited CaO, or dry it with Na2CO3, then fractionally distil it. Redistil it from Na. It is further purified by fractional crystallisation from the melt in dry air. Peroxides and aldehydes can be removed by prior washing with ferrous sulfate and water, followed by distillation under nitrogen from 2,4-dinitrophenylhydrazine, using a short fractionating column: water distils as the azeotrope. Dry cyclohexanol is very hygroscopic. The 3,4-dinitrobenzoate has m 111-112o (EtOH or aqueous EtOH) It has TOXIC vapours. [Beilstein 6 III 10, 6 IV 20.]
Incompatibilities
Forms explosive mixture in air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoa- cids, epoxides. Attacks some plastics.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera- tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Cyclohexanol
| Melting point: | 20-22 °C (lit.) |
| Boiling point: | 160-161 °C (lit.) |
| Density | 0.948 g/mL at 25 °C (lit.) |
| vapor density | 3.5 (vs air) |
| vapor pressure | 0.98 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 67 °C |
| storage temp. | Store below +30°C. |
| solubility | 40g/l |
| form | Liquid After Melting |
| pka | 16 |
| color | Clear colorless |
| Odor | Like camphor. |
| Relative polarity | 0.509 |
| PH | 6.5 (40g/l, H2O, 20℃) |
| explosive limit | 2-11.2%(V) |
| Water Solubility | 3.6 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,2725 |
| BRN | 906744 |
| Henry's Law Constant | 16.9 at 50.00 °C, 34.4 at 60.00 °C (headspace-GC, Hovorka et al., 2002) |
| Exposure limits | TLV-TWA 200 mg/m3 (50 ppm) (ACGIH);
IDLH 3500 ppm (NIOSH). |
| Dielectric constant | 15.0(25℃) |
| Stability: | Stable. Incompatible with oxidizing agents. Reacts violently with oxidizing agents such as hydrogen peroxide and nitric acid, even at room temperature, to form an explosive material. Hygroscopic. Combustible. |
| CAS DataBase Reference | 108-93-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyclohexanol(108-93-0) |
| EPA Substance Registry System | Cyclohexanol (108-93-0) |
Safety information for Cyclohexanol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cyclohexanol
Cyclohexanol manufacturer
Leo Chemo Plast Pvt. Ltd.
Pragati International
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Cyclohexanol extrapure CAS 108-93-0View Details
Cyclohexanol extrapure CAS 108-93-0View Details
108-93-0 -
 Cyclohexanol CASView Details
Cyclohexanol CASView Details -
 CyclohexanolView Details
CyclohexanolView Details
108-93-0 -
 Industrial Cyclohexanol ChemicalView Details
Industrial Cyclohexanol ChemicalView Details
108-93-0 -
 Cyclohexanol(108-93-0) , >99% Purity,200 Litres Drum ,Used as StabilizerView Details
Cyclohexanol(108-93-0) , >99% Purity,200 Litres Drum ,Used as StabilizerView Details
108-93-0 -
 Cyclohexanol (108-93-0), 99% Purity, 200 Litres Drum Used as Chemical IntermediateView Details
Cyclohexanol (108-93-0), 99% Purity, 200 Litres Drum Used as Chemical IntermediateView Details
108-93-0 -
 CYCLOHEXANOLView Details
CYCLOHEXANOLView Details
108-93-0 -
 CyclohexanolView Details
CyclohexanolView Details
108-93-0
