NICKEL(II) BROMIDE TRIHYDRATE
Synonym(s):Nickel bromide;Nickel bromide (Br2 Ni);Nickel dibromide;Nickel dibromide hydrate;Nickelous bromide
- CAS NO.:7789-49-3
- Empirical Formula: Br2H2NiO
- Molecular Weight: 236.52
- MDL number: MFCD00149807
- EINECS: 677-463-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
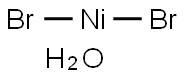
What is NICKEL(II) BROMIDE TRIHYDRATE?
Chemical properties
(1) Brownish-yellow solid or yellow, lustrous scales.(2) Deliquescent, greenish scales; loses 3H2O at 300C. Soluble in water, alcohol, ether, and ammonium hydroxide.
Chemical properties
The green hexahydrate NiBr2-6H2O readily evolves water; crystallization from aqueous solutions above 29° yields the trihydrate NiBr2-3H2O. The hexahydrate can be dehydrated to a dihydrate over concentrated sulphuric acid at 5°. The environment of the nickel atoms in NiBr2-6H20 is 2Br and 4H20 and in NiBr2-2H20 4Br and 2H20 ; in both cases the Ni-Br distance is 2.6 and the Ni-O distance 2.0 ?.
The Uses of NICKEL(II) BROMIDE TRIHYDRATE
It is used in Soporific and sedatives pharmaceutical Industry, Photosensitive industry, Analytical Chemistry, and also electrolyte in high energy battery.
Preparation
Anhydrous NiBr2 is most conveniently prepared by the action of bromine on nickel, either at red heat or in ethereal solution at room temperature74 or by dehydration of the hexahydrate at about 140°. It crystallizes with the CdCl2 type structure, the nickel atoms being surrounded octahedrally by bromine atoms at 2.57 ?.
Hazard
See nickel.
Properties of NICKEL(II) BROMIDE TRIHYDRATE
| Melting point: | 963 °C(lit.) |
| Density | 5.098 g/mL at 25 °C(lit.) |
| form | powder |
| Water Solubility | Soluble in water, alcohol |
| Sensitive | Hygroscopic |
| Merck | 14,6502 |
| Exposure limits | ACGIH: TWA 0.1 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
| CAS DataBase Reference | 7789-49-3(CAS DataBase Reference) |
Safety information for NICKEL(II) BROMIDE TRIHYDRATE
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory H341:Germ cell mutagenicity H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for NICKEL(II) BROMIDE TRIHYDRATE
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran

You may like
-
 Nickel(II) bromide trihydrate CAS 7789-49-3View Details
Nickel(II) bromide trihydrate CAS 7789-49-3View Details
7789-49-3 -
 Nickel(II) bromide trihydrate CAS 7789-49-3View Details
Nickel(II) bromide trihydrate CAS 7789-49-3View Details
7789-49-3 -
 Nickel(II) bromide trihydrate CAS 7789-49-3View Details
Nickel(II) bromide trihydrate CAS 7789-49-3View Details
7789-49-3 -
 Avibactam sodium seriesView Details
Avibactam sodium seriesView Details
1192651-49-2 -
 Avibactam seriesView Details
Avibactam seriesView Details
1192651-80-1 -
 Avibactam Sodium SaltView Details
Avibactam Sodium SaltView Details
1192491-61-4 -
 Avibactam intermediateView Details
Avibactam intermediateView Details
1171080-45-7 -
 Relibatan intermediateView Details
Relibatan intermediateView Details
1416134-63-8
