Cobalt carbonyl
Synonym(s):Cobalt tetracarbonyl dimer;Di-μ-carbonylhexacarbonyldicobal;Dicobalt octacarbonyl;Octacarbonyldicobalt
- CAS NO.:10210-68-1
- Empirical Formula: C8Co2O8+4
- Molecular Weight: 341.95
- MDL number: MFCD00016024
- EINECS: 233-514-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
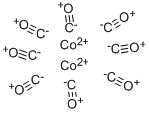
What is Cobalt carbonyl?
Description
Cobalt carbonyl is a pyrophoric (spontaneously flammable in air), red-orange (when pure) to darkbrown crystalline solid. Molecular weight =341.94; Boilingpoint =(decomposes) 52℃; Freezing/Melting point =(decomposes) 50.5℃; Vapor pressure = 0.7 mmHg at 25℃.Hazard Identification (based on NFPA-704 M RatingSystem): Health 3, Flammability 3, Reactivity 2 .Insoluble in water.
Chemical properties
red-orange to dark red crystals or flakes
Chemical properties
Cobalt carbonyl is a pyrophoric (spontaneously flammable in air), red-orange (when pure) to dark-brown crystalline solid.
Physical properties
Orange crystals; density 1.78 g/cm3; melts at 5l°C; decomposes above this temperature; insoluble in water; soluble in most organic solvents including alcohol, ether, carbon disulfide.
The Uses of Cobalt carbonyl
- Cobalt carbonyl [Co2(CO)8] is commonly used as a catalyst in the hydroformylation (oxo reaction) of alkenes.
- Along with pyridine, it can be used as a catalyst in the carboxylation of alkenes into corresponding acids and esters.
- It is employed as a key precursor in the preparation of cobalt platinum (CoPt3), cobalt sulfide (Co3S4) and cobalt selenide (CoSe2) nanocrystals.
- It is also used as a reagent in Pauson-Khand cyclizations and Nicholas reaction.
The Uses of Cobalt carbonyl
Cobalt octacarbonyl is used as a catalyst in the Oxo process. It also is used as a catalyst for hydrogenation, isomerization, hydrosilation and polymerization reactions. The compound is also a source of producing pure cobalt metal and its purified salts.
The Uses of Cobalt carbonyl
It is used as a catalyst in many organicconversion reactions, which include hydrogenation,isomerization, hydroformylation,polymerization, and carbonylation.
The Uses of Cobalt carbonyl
The use of dicobalt octacarbonyl as a catalyst in a variety of organic syntheses has led to the study of an extensive and important organometallic chemistry of cobalt.
Preparation
Cobalt octacarbonyl is prepared by the reaction of finely divided cobalt with carbon monoxide under pressure:
2Co + 8CO → Co2(CO)8
The compound may be prepared in a similar way from cobalt(II) iodide. Also, it may be prepared by thermal decomposition of cobalt carbonyl hydride:
2HCo(CO)4 → Co2(CO)8 + H2
Hazard
Toxic by ingestion and inhalation.
Health Hazard
Dicobalt octacarbonyl exhibits moderate toxicityby inhalation route and somewhatlower toxicity by intraperitoneal and oralroutes. However, it is much less toxicthan nickel tetracarbonyl or iron pentacarbonyl.A 2-hour LC50 value in mice isreported as 27 mg/m3 (Lewis 1996). Anoral LD50 value in rats is within the rangeof 750–800 mg/kg. It decomposes, evolvingtoxic carbon monoxide.
Safety Profile
Poison by inhalation and intraperitoneal routes. Questionable carcinogen. Decomposes in air to form a product that ignites spontaneously in air. "hen heated to decomposition it emits acrid smoke and fumes. See also CARBONYLS and COBALT COMPOUNDS.
Potential Exposure
This material is used as a catalyst for a number of reactions. It is also used in antiknock gasoline and for high-purity cobalt salts.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
storage
Color Code—Red Stripe: Flammability Hazard:Store separately from all other flammable materials. Priorto working with this chemical you should be trained on itsproper handling and storage. Decomposes on exposure toair or heat; stable in atmosphere of hydrogen and carbonmonoxide. Store in airtight, unbreakable containers in acool, well-ventilated area away from strong oxidizers andacids.
Shipping
UN3124 Toxic solids, self-heating, n.o.s., Hazard Class: 6.1; 6.1-Poisonous materials, 4.2-Spontaneously combustible material. Technical Name Required. UN3190 Self-heating solid, inorganic, Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material, Technical Name Required. UN1325 Flammable solid, organic, n.o.s. Hazard Class: 4.1; Labels: 4.1-Flammable solid
Purification Methods
It forms orange-brown crystals on recrystallisation from n-hexane under a carbon monoxide atmosphere [Ojima et al. J Am Chem Soc 109 7714 1987; see also Hileman in Preparative Inorganic Reactions, Ed. Jolly, Vol 1 p 101 1987].
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Decomposes on exposure to air or heat (@ ~52°C) producing toxic fumes of cobalt and oxides of carbon
Properties of Cobalt carbonyl
| Melting point: | 51-52 °C |
| Boiling point: | 52°C |
| Density | 1.81 |
| vapor pressure | 9.333-200Pa at 15-25℃ |
| Flash point: | -13 °C |
| storage temp. | 2-8°C |
| solubility | Dichloromethane (Slightly), Methanol (Slightly) |
| form | crystal |
| Specific Gravity | 1.73 |
| color | dark orange |
| Water Solubility | Insoluble in water. Soluble in alcohol, ether and carbon disulfideSoluble in ether, naphtha and carbon disulfide. Slightly soluble in alcohol. Insoluble in water. |
| Sensitive | Air Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| Merck | 13,3112 |
| Exposure limits | TLV-TWA: 0.1 mg/m3 as Co (ACGIH) PEL-TWA: 0.1 mg/m3 as Co (OSHA). |
| Stability: | Air sensitive |
| CAS DataBase Reference | 10210-68-1(CAS DataBase Reference) |
| EPA Substance Registry System | Cobalt carbonyl (10210-68-1) |
Safety information for Cobalt carbonyl
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H251:Self-heating substances and mixtures H302:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H330:Acute toxicity,inhalation H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P235:Keep cool. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Cobalt carbonyl
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 3-Nitrobenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![Carbonylchlorohydrido[6-(di-t-butylphosphinomethyl)-2-(N,N-diethylaminomethyl)pyridine]ruthenium(II), min. 98% (Milstein Catalyst Precursor)](https://img.chemicalbook.in/CAS/20211123/GIF/863971-62-4.gif)
You may like
-
 Octacarbonyldicobalt, Stabilized with 1 to 5% hexane CAS 10210-68-1View Details
Octacarbonyldicobalt, Stabilized with 1 to 5% hexane CAS 10210-68-1View Details
10210-68-1 -
 Octacarbonyldicobalt, Stabilized with 1 to 5% hexane CAS 10210-68-1View Details
Octacarbonyldicobalt, Stabilized with 1 to 5% hexane CAS 10210-68-1View Details
10210-68-1 -
 Octacarbonyldicobalt, Stabilized with 1 to 5% hexane CAS 10210-68-1View Details
Octacarbonyldicobalt, Stabilized with 1 to 5% hexane CAS 10210-68-1View Details
10210-68-1 -
 Dicobalt Octacarbonyl (stabilized with 1-5% Hexane) CAS 10210-68-1View Details
Dicobalt Octacarbonyl (stabilized with 1-5% Hexane) CAS 10210-68-1View Details
10210-68-1 -
 Dicobalt octacarbonyl 94% stab. with 1-5 hexane CAS 10210-68-1View Details
Dicobalt octacarbonyl 94% stab. with 1-5 hexane CAS 10210-68-1View Details
10210-68-1 -
 Cobalt carbonyl CAS 10210-68-1View Details
Cobalt carbonyl CAS 10210-68-1View Details
10210-68-1 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0