Clopidogrel
- CAS NO.:113665-84-2
- Empirical Formula: C16H16ClNO2S
- Molecular Weight: 321.82
- MDL number: MFCD05662337
- EINECS: 601-269-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
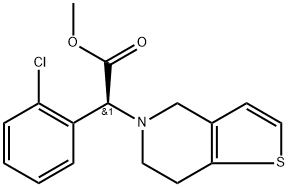
What is Clopidogrel?
Absorption
A 75mg oral dose of clopidogrel is 50% absorbed from the intestine. Clopidogrel can be taken with or without food. A meal decreases the AUC of the active metabolite by 57%. The active metabolite of clopidogrel reaches a maximum concentration after 30-60 minutes. Clopidogrel reached a Cmax of 2.04±2.0ng/mL in 1.40±1.07h.
The AUC for a 300mg oral dose of clopidogrel was 45.1±16.2ng*h/mL for poor metabolizers, 65.6±19.1ng*h/mL for intermediate metabolizers, and 104.3±57.3ng*h/mL for extensive metabolizers. The Cmax was 31.3±13ng/mL for poor metabolizers, 43.9±14ng/mL for intermediate metabolizers, and 60.8±34.3ng/mL for extensive metabolizers.
Toxicity
A single dose of clopidogrel at 1500-2000mg/kg was lethal to mice and rats while 3000mg/kg was lethal to baboons. Symptoms of overdose include vomiting, breathing difficulty, gastrointestinal hemorrhage, and prostration. Clopidogrel is irreversibly bound to platelets for their lifetime, which is approximately 11 days. Overdoses of clopidogrel can be treated with platelet transfusions to restore clotting ability.
Description
Clopidogrel was launched in the US as a potent inhibitor of platelet aggregation for the preventive management of secondary ischemic events, including MI, stroke and vascular deaths. Clopidogrel can be synthesized in 4 steps (including an optical resolution to the S active enantiomer) from 2-(2- ch1orophenyl)-glycine, the key step being the cyclization to thienopyridine with formaldehyde and acetic acid. Clopidogrel belongs to the original chemical class of Ticlopidine, but shows fewer side effects (in particular, bone-marrowsuppressing effects) at the dosage generally used. Like Ticlopidine, it is an Adenosine diphosphate (ADP) antagonist acting at the purinergic P2y receptor. In in vivo experiments with rabbits, Clopidogrel shows a maximal antiaggregant effect at 20mg/kg po, reducing adhesion of platelets to the vascular subendothelium ; moreover, it reduces myointimal thickening occuring after endothelial injury of rat carotid artery. Clopigrel does not affect platelet aggregation in vitro ; actually, its in vivo activity is highly dependent on hepatic metabolism. The results of a CAPRIE trial (Clopidogrel versus Aspirin in patients at risk of ischemic events) demonstrated that Clopidogrel was well tolerated and more effective than aspirin.
Description
Clopidogrel, sold under the brand name Plavix, is commonly known as a “blood thinner”. It prevents blood from clotting in patients who are vulnerable to stroke, heart attack, and other vascular diseases. Clopidogrel is actually a prodrug—its thiophene ring hydrolyzes in vivo to the active metabolite. An improved synthesis of the chiral α-amino ester moiety in clopidogrel was described in the?June 13, 2011, edition of Noteworthy Chemistry.
Originator
Sanofi (France)
The Uses of Clopidogrel
anthelmintic, antiparasitic, antimite
The Uses of Clopidogrel
Sertraline metabolite
Indications
Clopidogrel is indicated to reduce the risk of myocardial infarction for patients with non-ST elevated acute coronary syndrome (ACS), patients with ST-elevated myocardial infarction, and in recent MI, stroke, or established peripheral arterial disease,
Background
Clopidogrel is a prodrug of a platelet inhibitor used to reduce the risk of myocardial infarction and stroke. Clopidogrel is indicated to reduce the risk of myocardial infarction for patients with non-ST elevated acute coronary syndrome (ACS), patients with ST-elevated myocardial infarction, and in recent MI, stroke, or established peripheral arterial disease,
It has been shown to be superior to aspirin in reducing cardiovascular outcomes in patients with cardiovascular disease and provides additional benefit to patients with acute coronary syndromes already taking aspirin.
Clopidogrel was granted FDA approval on 17 November 1997.
Definition
ChEBI: Clopidogrel is a thienopyridine that is 4,5,6,7-tetrahydrothieno[3,2-c]pyridine in which the hydrogen attached to the nitrogen is replaced by an o-chlorobenzyl group, the methylene hydrogen of which is replaced by a methoxycarbonyl group (the S enantiomer). A P2Y12 receptor antagonist, it is used to inhibit blood clots and prevent heart attacks. It has a role as a platelet aggregation inhibitor, an anticoagulant and a P2Y12 receptor antagonist. It is a thienopyridine, a member of monochlorobenzenes and a methyl ester. It is functionally related to a ticlopidine.
brand name
Plavix (Sanofi Aventis);Plavix, Iscover.
General Description
Clopidogrel, methyl (+)-(S)-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetate sulfate (Plavix), is useful for the preventativemanagement of secondary ischemic events, including myocardialinfarction, stroke, and vascular deaths. It may beclassified as a thienopyridine because of its heterocyclicsystem. Several agents possessing this system have beenevaluated as potential antithrombotic agents. These agentshave a unique mechanism, in that they inhibit the purinergicreceptor located on platelets. Normally, nucleotides act asagonists on these receptors, which include the P2Y type.Two P2Y receptor subtypes (P2Y1 and P2Y2) found onplatelets, when stimulated by adenosine diphosphate (ADP),cause platelet aggregation.
Pharmacokinetics
Clopidogrel is a prodrug of a platelet inhibitor used to reduce the risk of myocardial infarction and stroke. It has a long duration of action as it is taken once daily and a large therapeutic window as it is given in doses of 75-300mg daily.
Clinical Use
Clopidogrel acts as an antagonistto the P2Y2 receptor. It is probably a prodrug that requiresmetabolic activation, because in vitro studies do not interferewith platelet aggregation. Although platelet aggregationis not normally seen in the first 8 to 11 days after administrationto a patient, the effect lasts for several days after thedrug therapy is discontinued. Unlike other thienopyridinescurrently used, clopidogrel does not seriously reduce thenumber of white cells in the blood, and therefore, routinemonitoring of the white blood cell count is not necessaryduring treatment.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: antiplatelet effect possibly reduced by
erythromycin.
Anticoagulants: enhanced anticoagulant effect with
coumarins and phenindione; manufacturer advises to
avoid with warfarin.
Heparin: increased risk of bleeding.
Antidepressants: antiplatelet effect possibly reduced
by fluoxetine, fluvoxamine and moclobemide.
Anti-diabetics: avoid with repaglinide if possible due
to increased repaglinide exposure.
Antiepileptics: antiplatelet effect possibly reduced by
carbamazepine and oxcarbazepine.
Antifungals: antiplatelet effect possibly reduced
by fluconazole, itraconazole, ketoconazole and
voriconazole.
Antivirals: antiplatelet effect possibly reduced by
etravirine.
Statins: concentration of rosuvastatin increased,
maximum rosuvastatin dose is 20 mg.
Ulcer healing drugs: antiplatelet effect possibly
reduced by cimetidine, lansoprazole, pantoprazole
and rabeprazole; antiplatelet effect reduced by
omeprazole and esomeprazole - avoid concomitant
use if possible.
Metabolism
85-90% of an oral dose undergoes first pass metabolism by carboxylesterase 1 in the liver to an inactive carboxylic acid metabolite. about 2% of clopidogrel is oxidized to 2-oxoclopidogrel. This conversion is 35.8% by CYP1A2, 19.4% by CYP2B6, and 44.9% by CYP2C19 though other studies suggest CYP3A4, CYP3A5, and CYP2C9 also contribute. 2-oxoclopidogrel is further metabolized to the active metabolite. This conversion is 32.9% by CYP2B6, 6.79% by CYP2C9, 20.6% by CYP2C19, and 39.8% by CYP3A4.
Metabolism
Clopidogrel is a prodrug and is extensively metabolised in
the liver, mainly to the inactive carboxylic acid derivative;
metabolism is mediated by cytochrome P450 isoenzymes
including CYP3A4 and CYP2B6, CYP1A2, CYP1A1,
and CYP2C19. The active metabolite appears to be a
thiol derivative
Clopidogrel and its metabolites are excreted in urine and
in faeces; about 50% of an oral dose is recovered from the
urine and about 46% from the faeces.
Properties of Clopidogrel
| Boiling point: | 423.7±45.0 °C(Predicted) |
| alpha | D20 +51.52° (c = 1.61 in methanol) |
| Density | 1.317±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: 50mg/ml in DMSO |
| form | Oil |
| pka | 4.56±0.20(Predicted) |
| color | Colorless to light yellow |
| InChI | InChI=1/C16H16ClNO2S/c1-20-16(19)15(12-4-2-3-5-13(12)17)18-8-6-14-11(10-18)7-9-21-14/h2-5,7,9,15H,6,8,10H2,1H3/t15-/s3 |
| CAS DataBase Reference | 113665-84-2(CAS DataBase Reference) |
Safety information for Clopidogrel
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Clopidogrel
| InChIKey | GKTWGGQPFAXNFI-UJHUVDBMNA-N |
| SMILES | [C@@H](C1C=CC=CC=1Cl)(N1CCC2SC=CC=2C1)C(=O)OC |&1:0,r| |
Clopidogrel manufacturer
Sainor Life Sciences Pvt Ltd
Zenfold Sustainable Technologies (ZST)
Stellar Chemical Laboratories Pvt., Ltd.
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 113665-84-2 CLOPIDOGREL 50% DC GRANULES 98%View Details
113665-84-2 CLOPIDOGREL 50% DC GRANULES 98%View Details
113665-84-2 -
 Clopidogrel 113665-84-2 98%View Details
Clopidogrel 113665-84-2 98%View Details
113665-84-2 -
 Clopidogrel 98%View Details
Clopidogrel 98%View Details
113665-84-2 -
 113665-84-2 98%View Details
113665-84-2 98%View Details
113665-84-2 -
 113665-84-2 98%View Details
113665-84-2 98%View Details
113665-84-2 -
 Clopidogrel 99%View Details
Clopidogrel 99%View Details -
 Clopidogrel 95% CAS 113665-84-2View Details
Clopidogrel 95% CAS 113665-84-2View Details
113665-84-2 -
 Clopidogrel 113665-84-2 99%View Details
Clopidogrel 113665-84-2 99%View Details
113665-84-2