Tetrahydrothiophene
Synonym(s):Tetrahydrothiophene;THT;Tetramethylene sulfide;Thiolane;Thiophane
- CAS NO.:110-01-0
- Empirical Formula: C4H8S
- Molecular Weight: 88.17
- MDL number: MFCD00005476
- EINECS: 203-728-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
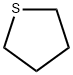
What is Tetrahydrothiophene?
Chemical properties
Tetrahydrothiophene is cyclic thioalkane, namely, saturated analog of thiophene. It is a colorless transparent volatile liquid, insoluble in water, miscible in ethanol, ether, benzene, acetone. It has a strong unpleasant odor, is stable and not easily emitted, and can be smelled in the air at 0.01PPm. It is not corrosive to gas equipment, transportation pipeline gaskets and other materials, and does not produce habitual blunting of the human sense of smell, so it is used as an odorant in LPG and natural gas, outlawing the original use of ethyl mercaptan and other odor-assigning agents.
The Uses of Tetrahydrothiophene
Tetrahydrothiophene is a solvent and a fuel gas odorant used in fire safety stench warning systems in underground mines. Tetrahydrothiophene was also used as an organocatalyst for the reaction between enynediones and benzoic acid to afford highly substituted furfuryl alcohol benzoates.
The Uses of Tetrahydrothiophene
Tetrahydrothiophene is used as pharmaceutical and agrochemical intermediates.
Preparation
Tetrahydrothiophene is prepared by the reaction of hydrogen sulfide and furan in the vapor phase at 400°C. This vapor-phase reaction is catalyzed by alumina(Al2O3) and other heterogenous acid catalysts.
Definition
ChEBI: Tetrahydrothiophene is a saturated organic heteromonocyclic parent and a member of tetrahydrothiophenes.
General Description
A water-white liquid. About the same density as water and insoluble in water. Flash point near 20°F. Vapors heavier than air. Used as a solvent and to make other chemicals.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Organosulfides, such as Tetrahydrothiophene, are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Slow addition of hydrogen peroxide to the thiophene resulted in explosions on three occasions, Chem. Eng. News, 1974, 52(39), 3.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Mildly toxic by inhalation. Flammable liquid. Potentially explosive reaction with hydrogen peroxide. When heated to decomposition it emits toxic fumes of SOx.
Purification Methods
The crude material is purified by crystallisation of the mercuric chloride complex to a constant melting point. It is then regenerated, washed, dried, and fractionally distilled. [Whitehead et al. J Am Chem Soc 73 3632 1951.] It has been dried over Na2SO4 and distilled in a vacuum [Roberts & Friend J Am Chem Soc 108 7204 1986]. [Beilstein 17 I 5, 17 II 15, 17 III/IV 34, 17/1 V 36.]
Properties of Tetrahydrothiophene
| Melting point: | -96 °C (lit.) |
| Boiling point: | 119 °C (lit.) |
| Density | 1 g/mL at 25 °C (lit.) |
| vapor pressure | 18 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 55 °F |
| storage temp. | Store below +30°C. |
| solubility | immiscible |
| form | liquid |
| color | Colorless to Almost colorless |
| Specific Gravity | 1.002 (20/4℃) |
| Odor | Smells of rotting eggs |
| PH | 7 (H2O, 20℃) |
| explosive limit | 1.1-12.1%(V) |
| Odor Threshold | 0.00062ppm |
| Water Solubility | immiscible |
| Merck | 14,9218 |
| BRN | 102392 |
| Dielectric constant | 8.6099999999999994 |
| Stability: | Highly flammable. Vapour-air mixtures explosive in some proportions; note low flash point and fairly wide explosion limit range. Heavier than air, so potentially explosive mixtures may travel considerable distances to source of ignition. |
| CAS DataBase Reference | 110-01-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Thiophene, tetrahydro-(110-01-0) |
| EPA Substance Registry System | Tetrahydrothiophene (110-01-0) |
Safety information for Tetrahydrothiophene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Tetrahydrothiophene
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![4,5,6,7-Tetrahydrothieno[3,2-c]pyridine](https://img.chemicalbook.in/CAS/GIF/54903-50-3.gif)

You may like
-
 110-01-0 98%+View Details
110-01-0 98%+View Details
110-01-0 -
 Tetrahydrothiophene CAS 110-01-0View Details
Tetrahydrothiophene CAS 110-01-0View Details
110-01-0 -
 Tetrahydrothiophene CAS 110-01-0View Details
Tetrahydrothiophene CAS 110-01-0View Details
110-01-0 -
 Tetrahydrothiophene CAS 110-01-0View Details
Tetrahydrothiophene CAS 110-01-0View Details
110-01-0 -
 Tetrahydrothiophene Chemical Powder, 99%, For LabView Details
Tetrahydrothiophene Chemical Powder, 99%, For LabView Details
110-01-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
