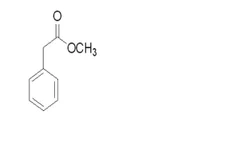
Methyl phenylacetate
- CAS NO.:101-41-7
- Empirical Formula: C9H10O2
- Molecular Weight: 150.17
- MDL number: MFCD00008453
- EINECS: 202-940-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 11:21:09
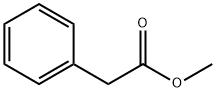
What is Methyl phenylacetate?
Description
Methyl phenylacetate belongs to an ester compound form between the methanol and phenylacetate. It chemical formula is C6H5CH2COOCH3. It is generally found in brandy, capsicum, honey, pepper, some wine, coca and coca products. It is mainly used as a flavouring agent, and is also supplemented into perfumes to enhance honey scents. It can also be used as the precursor for the manufacture of synthetic perfumes. In addition, as an acylating agent, it can participate in the enantioselective acylation reaction of beta-lactam intermediate catalyzed by the penicillin G amidase.
Chemical properties
Methyl phenylacetate is a clear colorless liquid that is only slightly soluble in water, but very soluble in most organic solvents. It has a strong odor similar to honey. The odor is so strong that recommended smelling is of a solution with 10% or less methyl phenyl acetate. This compound also naturally occurs in brandy, capsicum, coffee, honey, pepper and some wine.
Occurrence
Reported found in cocoa, coffee, strawberry, pineapple, pepper, hop oil, cognac, peanut, honey, starfruit, Bourbon vanilla, mountain papaya, roasted chicory root and rooibus tea (Aspalathus linearis).
The Uses of Methyl phenylacetate
Methyl phenylacetate is utilized for partition coefficient measurement experiments. It is mainly used in the flavor industry and in perfumes to impart honey scents. Further, it acts as a precursor to prepare synthetic perfumes. It acts as an acylating agent and involved in the enantioselective acylation of beta-lactam intermediate using penicillin G amidase.
Preparation
Methyl phenylacetate is synthesized from phenylacetonitrile by hydrolysis and esterification. Put methanol into a dry glass-lined reaction pot, stir and cool to below 30°C, add sulfuric acid dropwise, heat up to 90°C after adding, and start adding phenylacetonitrile dropwise, control the temperature at about 95°C, and finish adding in 1.5h. After reacting at 95-100 °C for 6 h, it was cooled to below 40 °C and diluted with water equivalent to about 0.6 times the reaction solution. The acid water was separated by standing, and a saturated sodium carbonate solution was added for neutralization and washing, and the aqueous layer was discarded. It is dehydrated with anhydrous calcium chloride and fractionated under reduced pressure to obtain methyl phenylacetate with a yield of 80%.
What are the applications of Application
Methyl Phenylacetate is used as a reagent in the synthesis of various organic reactions, one of which is the synthesis of Vulpinic Acid; a lichen metabolite with anti-inflammatory properties. It is also used in the formulation of edible flavors, for the preparation of honey, chocolate, tobacco and other flavors; it can also be used in daily chemical flavors for the preparation of rose, oriental flavors and other flavors. IFRA has no restrictions.
Definition
ChEBI: Methyl benzeneacetate is a member of benzenes. It is a flavoring ingredient. Odoriferous constituent of many plants. It is generally found in brandy, capsicum, honey, pepper, some wine, coca and coca products.
Aroma threshold values
Detection: 25 ppb
Taste threshold values
Taste characteristics at 30 ppm: floral, fruity, honey, spice, waxy and sweet
Synthesis Reference(s)
Journal of the American Chemical Society, 93, p. 4919, 1971 DOI: 10.1021/ja00748a051
Tetrahedron Letters, 30, p. 2945, 1989 DOI: 10.1016/S0040-4039(00)99165-2
General Description
Methyl phenylacetate has been identified in the volatile fraction of beewax absolute oil, dried fruiting bodies of Boletus auripes, Chinese fermented black soybeans and peated malt.
Biochem/physiol Actions
Methyl phenylacetate undergoes decomposition on photolysis in methanol. Methyl phenylacetate acts as acylating agent and causes the enantioselective acylation of beta-lactam intermediate using penicillin G amidase. Methyl phenylacetate is the starting material in manufacture of synthetic perfumes.
Safety Profile
Moderately toxic by ingestion and skin contact. A skin irritant. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS.
Properties of Methyl phenylacetate
| Melting point: | 107-115 °C |
| Boiling point: | 218 °C (lit.) |
| Density | 1.066 g/mL at 20 °C (lit.) |
| vapor pressure | 16.9-75Pa at 20℃ |
| FEMA | 2733 | METHYL PHENYLACETATE |
| refractive index | n |
| Flash point: | 195 °F |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | neat |
| color | Colourless |
| Specific Gravity | 1.07 |
| Odor | at 10.00 % in dipropylene glycol. sweet floral honey spice waxy almond |
| Water Solubility | Miscible with water. |
| JECFA Number | 1008 |
| Merck | 14,7268 |
| BRN | 878795 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases. |
| CAS DataBase Reference | 101-41-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzeneacetic acid, methyl ester(101-41-7) |
| EPA Substance Registry System | Methyl phenylacetate (101-41-7) |
Safety information for Methyl phenylacetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H227:Flammable liquids H303:Acute toxicity,oral |
| Precautionary Statement Codes |
P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for Methyl phenylacetate
| InChIKey | CRZQGDNQQAALAY-UHFFFAOYSA-N |
Methyl phenylacetate manufacturer
Kaival Chemicals Private Limited
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 benzeneacetic acid methyl ester 98%View Details
benzeneacetic acid methyl ester 98%View Details
101-41-7 -
 Methyl phenylacetate 98%View Details
Methyl phenylacetate 98%View Details -
 Methyl phenylacetate 98%View Details
Methyl phenylacetate 98%View Details
101-41-7 -
 Methyl Phenylacetate CAS 101-41-7View Details
Methyl Phenylacetate CAS 101-41-7View Details
101-41-7 -
 Methyl phenyl acetate CAS 101-41-7View Details
Methyl phenyl acetate CAS 101-41-7View Details
101-41-7 -
 Liquid Methyl Phenyl Acetate, Packaging Type: Drum, Packaging Size: 200 KgView Details
Liquid Methyl Phenyl Acetate, Packaging Type: Drum, Packaging Size: 200 KgView Details
101-41-7 -
 Methyl Phenyl Acetate, Packaging Size: 25 KgView Details
Methyl Phenyl Acetate, Packaging Size: 25 KgView Details
101-41-7 -
 Methyl Phenyl Acetate, Packaging Size: LooseView Details
Methyl Phenyl Acetate, Packaging Size: LooseView Details
101-41-7
