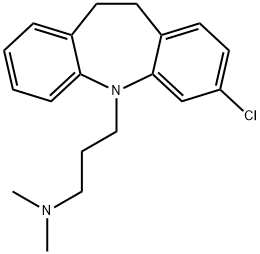
Clomipramine
- CAS NO.:303-49-1
- Empirical Formula: C19H23ClN2
- Molecular Weight: 314.85
- MDL number: MFCD00242755
- EINECS: 206-144-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
What is Clomipramine?
Absorption
Well absorbed from the GI tract following oral administration. Bioavailability is approximately 50% orally due to extensive first-pass metabolism. Bioavailability is not affected by food. Peak plasma concentrations occurred 2-6 hours following oral administration of a single 50 mg dose. The peak plasma concentration ranged from 56 ng/mL to 154 mg/mL (mean, 92 ng/mL). There are large interindividual variations in plasma concentrations occur, partly due to genetic differences in clomipramine metabolism. On average, steady state plasma concentrations are achieved in 1-2 weeks following multiple dose oral administration. Smoking appears to lower the steady-state plasma concentration of clomipramine, but not its active metabolite desmethylclomipramine.
Toxicity
Signs and symptoms vary in severity depending upon factors such as the amount of drug absorbed, the age of the patient, and the time elapsed since drug ingestion. Critical manifestations of overdose include cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic toxicity. In U.S. clinical trials, 2 deaths occurred in 12 reported cases of acute overdosage with Anafranil either alone or in combination with other drugs. One death involved a patient suspected of ingesting a dose of 7000 mg. The second death involved a patient suspected of ingesting a dose of 5750 mg. Side effects include: sedation, hypotension, blurred vision, dry mouth, constipation, urinary retention, postural hypotension, tachycardia, hypertension, ECG changes, heart failure, impaired memory and delirium, and precipitation of hypomanic or manic episodes in bipolar depression. Withdrawal symptoms include gastrointestinal disturbances, anxiety, and insomnia.
Originator
Anafranil,Ciba Geigy,Switz.,1968
The Uses of Clomipramine
Antidepressan.
Background
Clomipramine, the 3-chloro analog of imipramine, is a dibenzazepine-derivative tricyclic antidepressant (TCA). TCAs are structurally similar to phenothiazines. They contain a tricyclic ring system with an alkyl amine substituent on the central ring. In non-depressed individuals, clomipramine does not affect mood or arousal, but may cause sedation. In depressed individuals, clomipramine exerts a positive effect on mood. TCAs are potent inhibitors of serotonin and norepinephrine reuptake. Tertiary amine TCAs, such as clomipramine, are more potent inhibitors of serotonin reuptake than secondary amine TCAs, such as nortriptyline and desipramine. TCAs also down-regulate cerebral cortical β-adrenergic receptors and sensitize post-synaptic serotonergic receptors with chronic use. The antidepressant effects of TCAs are thought to be due to an overall increase in serotonergic neurotransmission. TCAs also block histamine-H1 receptors, α1-adrenergic receptors and muscarinic receptors, which accounts for their sedative, hypotensive and anticholinergic effects (e.g. blurred vision, dry mouth, constipation, urinary retention), respectively. See toxicity section below for a complete listing of side effects. Clomipramine may be used to treat obsessive-compulsive disorder and disorders with an obsessive-compulsive component (e.g. depression, schizophrenia, Tourette’s disorder). Unlabeled indications include panic disorder, chronic pain (e.g. central pain, idiopathic pain disorder, tension headache, diabetic peripheral neuropathy, neuropathic pain), cataplexy and associated narcolepsy, autistic disorder, trichotillomania, onchophagia, stuttering, premature ejaculation, and premenstrual syndrome. Clomipramine is rapidly absorbed from the gastrointestinal tract and demethylated in the liver to its primary active metabolite, desmethylclomipramine.
Indications
May be used to treat obsessive-compulsive disorder and disorders with an obsessive-compulsive component (e.g. depression, schizophrenia, Tourette’s disorder). Unlabeled indications include: depression, panic disorder, chronic pain (e.g. central pain, idiopathic pain disorder, tension headache, diabetic peripheral neuropathy, neuropathic pain), cataplexy and associated narcolepsy (limited evidence), autistic disorder (limited evidence), trichotillomania (limited evidence), onchophagia (limited evidence), stuttering (limited evidence), premature ejaculation, and premenstrual syndrome.
Definition
ChEBI: A dibenzoazepine that is 10,11-dihydro-5H-dibenzo[b,f]azepine which is substituted by chlorine at position 3 and in which the hydrogen attached to the nitrogen is replaced by a 3-(dimethylamino)propyl group. One of the more sedating tricyclic antidepressan s, it is used as the hydrochloride salt for the treatment of depression as well as obsessive-compulsive disorder and phobias.
Manufacturing Process
22.9 parts of 3-chloroiminodibenzyl are dissolved in 300 parts by volume of xylene, and 4 parts of sodium amide, pulverized and suspended in toluene,are added thereto while stirring and maintaining the whole under a nitrogen atmosphere. The xylene solution immediately turns dark colored, but upon crystallization of the sodium salt therefrom it becomes again light-colored. The reaction mixture is stirred for about 2 hours at 80°C until the development of ammonia has terminated. A solution of γ-dimethylaminopropyl chloride in toluene, prepared by setting free a corresponding amount of the free base from 17.4 parts of its hydrochloride salt by addition of aqueous sodium hydroxide solution in about 10% excess, extraction with toluene and drying for 2 hours over anhydrous sodium sulfate is added to the xylene solution containing the sodium salt mentioned above and the whole is stirred under reflux for 15 hours. Precipitated sodium chloride is filtered off and the filtrate is concentrated. The residue is diluted with ether, and the hydrochloride of 3- chloro-5-(γ-dimethylaminopropyl)-iminodibenzyl is precipitated by introducing dry, gaseous hydrogen chloride. It is filtered off under suction and purified by repeated recrystallization from acetone; the pure substance melts at 191.5°C to 192°C.
brand name
Anafranil (Tyco).
Therapeutic Function
Antidepressant
Mechanism of action
Clomipramine is different from the other TCAs, exhibiting preferential selectivity for inhibiting the reuptake of
5-HT at the presynaptic neuronal membrane. Its antidepressant mechanism of action as an inhibitor of the
5-HT transporter is reduced in vivo, however, because of the formation of its active metabolite,
N-desmethylclomipramine, which inhibits the reuptake of NE. As a result of its common structure with the
other TCAs, clomipramine shares the pharmacological and adverse-effect profile of the other TCAs.
The efficacy of clomipramine relative to the other TCAs in the treatment of obsessive-compulsive disorder
may be related to its potency in blocking 5-HT reuptake at the presynaptic neuronal membrane, suggesting a
dysregulation of 5-HT for the pathogenesis of obsessive-compulsive disorder. Clomipramine appears to
decrease the turnover of 5-HT in the CNS, probably because of a decrease in the release and/or synthesis of
5-HT.
Although in vitro studies suggest that clomipramine is approximately four times more potent than fluoxetine as
a 5-HT reuptake inhibitor, in vivo studies suggest the opposite. This difference has been attributed to the
relatively long elimination half-lives for fluoxetine and its principal serotonergic metabolite norfluoxetine. In
addition, metabolism of clomipramine to its N-desmethyl secondary amine metabolite decreases the potency
and selectivity of 5-HT -reuptake inhibition of clomipramine, but not fluoxetine.
Pharmacokinetics
Clomipramine, a tricyclic antidepressant, is the 3-chloro derivative of Imipramine. It was thought that tricyclic antidepressants work exclusively by inhibiting the re-uptake of the neurotransmitters norepinephrine and serotonin by nerve cells. However, this response occurs immediately, yet mood does not lift for around two weeks. It is now thought that changes occur in receptor sensitivity in the cerebral cortex and hippocampus. The hippocampus is part of the limbic system, a part of the brain involved in emotions. Presynaptic receptors are affected: α1 and β1 receptors are sensitized, α2 receptors are desensitized (leading to increased noradrenaline production). Tricyclics are also known as effective analgesics for different types of pain, especially neuropathic or neuralgic pain.
Pharmacokinetics
Clomipramine appears to be well absorbed from the GItract following oral administration, with an oral
bioavailability of approximately 50%, suggesting some first-pass metabolism. Food does not
appear to substantially affect its bioavailability. Clomipramine and its active metabolite,
N-desmethylclomipramine, exhibit nonlinear pharmacokinetics at 25 to 150 mg daily. At dosages exceeding
150 mg daily, their elimination half-lives may be considerably prolonged, allowing plasma concentrations of
both to accumulate, which may increase the incidence of plasma concentration-dependent adverse effects,
particularly seizures. Because of the relatively long elimination half-lives of clomipramine and
N-desmethylclomipramine, their steady-state plasma concentrations generally are achieved within
approximately 1 to 2 weeks. Plasma concentrations of N-desmethylclomipramine generally are greater than
those for chlomipramine at steady-state conditions. Clomipramine crosses the placenta and is distributed into
breast milk.
Clomipramine is primarily metabolized by CYP2D6 N-dealkylation to its pharmacologically active metabolite,
the 2- and 8-hydroxylated metabolites and their glucuronides, and clomipramine N-oxide.
N-dealkylation also involves CYP3A4, CYP2C19, CYP2C9, and CYP1A2. Like all the other secondary amine
TCAs, N-desmethylclomipramine is significantly more potent as an inhibitor of NE reuptake than clomipramine
is. Although N-desmethylclomipramine is pharmacologically active, its efficacy in obsessive-compulsive
disorder is not known. 8-Hydroxyclomipramine and 8-hydroxydesmethylclomipramine also are
pharmacologically active, but their clinical importance remains unknown. The hydroxylation and
N-demethylation of clomipramine highlight CYP2D6 polymorphism in healthy adults who were phenotyped as either extensive metabolizers or poor metabolizers of clomipramine. Interindividual variation in plasma
concentrations may be caused by genetic differences in the metabolism of the drug. In addition, CYP1A2 ring
hydroxylates clomipramine. Less than 1% of an oral dose of clomipramine was excreted unmetabolized into
the urine, with 8-hydroxyclomipramine glucuronide as the principal metabolite found in the urine. The effects
of renal clearance suggest that clomipramine and desmethylclomipramine should be decreased in patients
with renal impairment.
Clinical Use
Clomipramine is considered to be the most powerful antidepressant ever made. This dihydrodibenzazepine TCA, with actions on both the NE and 5-HT transporters, was the last of the major TCAs to come to market. Initially, the U.S. FDA regarded it as another “me-too” drug, and accordingly, they did not license it. Subsequently, however, it was licensed for the treatment of obsessive-compulsive disorders. Clomipramine differs from imipramine only by the addition of a 3-chloro group.
Side Effects
Male patients taking clomipramine should be informed of sexual dysfunction as a side effect associated with antidepressants having significant serotonergic activity. Sexual dysfunction in men appears as ejaculatory incompetence, ejaculatory retardation, decreased libido, or inability to obtain or maintain an erection. Sexual dysfunction is dose-related and may be treated by simply lowering the drug dose.
Metabolism
Extensively metabolized in the liver. The main active metabolite is desmethylclomipramine, which is formed by N-demethylation of clomipramine via CYP2C19, 3A4 and 1A2. Other metabolites and their glucuronide conjugates are also produced. Other metabolites of clomipramine include 8-hydroxyclomipramine formed via 8-hydroxylation, 2-hydroxyclomipramine formed via 2-hydroxylation, and clomipramine N-oxide formed by N-oxidation. Desmethylclomipramine is further metabolized to 8-hydroxydesmethylclomipramine and didesmethylclomipramine, which are formed by 8-hydroxylation and N-demethylation, respectively. 8-Hydroxyclomipramine and 8-hydroxydesmethylclomipramine are pharmacologically active; however, their clinical relevance remains unknown.
Properties of Clomipramine
| Melting point: | 189.5°C |
| Boiling point: | bp0.3 160-170° |
| Density | 1.0568 (rough estimate) |
| refractive index | 1.5749 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | H2O: 25 mg/mL |
| form | powder |
| pka | pKa 9.38(H2O) (Uncertain) |
| color | white to off-white |
| CAS DataBase Reference | 303-49-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Clomipramine(303-49-1) |
Safety information for Clomipramine
Computed Descriptors for Clomipramine
Clomipramine manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 303-49-1 99%View Details
303-49-1 99%View Details
303-49-1 -
 Clomipramine Ip Powder, 1 KgView Details
Clomipramine Ip Powder, 1 KgView Details
303-49-1 -
 ClomipramineView Details
ClomipramineView Details
303-49-1 -
 Medicine Grade Clomipramine PowderView Details
Medicine Grade Clomipramine PowderView Details
303-49-1 -
 Clomipramine 25 MgView Details
Clomipramine 25 MgView Details
303-49-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
