Clomipramine hydrochloride
Synonym(s):3-Chloro-10,11-dihydro-N,N-dimethyl-5H-dibenz[b,f]azepine-5-propanamine hydrochloride;Anafranil hydrochloride;Clomipramine hydrochloride
- CAS NO.:17321-77-6
- Empirical Formula: C19H24Cl2N2
- Molecular Weight: 351.31
- MDL number: MFCD00069234
- EINECS: 241-344-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
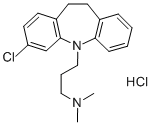
What is Clomipramine hydrochloride?
Description
Clomipramine hydrochloride is a tricyclic antidepressant, the 3-
Chemical properties
Off-White Solid
Originator
Anafranil,Ciba Geigy,Switz.,1968
The Uses of Clomipramine hydrochloride
Clomipramine hydrochloride is a selective ST, SLC6A2, and DAT inhibitor.
The Uses of Clomipramine hydrochloride
anti-depressant, serotonin uptake inhibitor
The Uses of Clomipramine hydrochloride
Potent, selective 5-HT uptake blocker
Definition
ChEBI: A hydrochloride resulting from the reaction of equimolar amounts of clomipramine and hydrogen chloride. One of the more sedating tricyclic antidepressants, it is used for the treatment of depression as well as obsessive-compulsive disorder and phobias.
Manufacturing Process
22.9 parts of 3-chloroiminodibenzyl are dissolved in 300 parts by volume of xylene, and 4 parts of sodium amide, pulverized and suspended in toluene,are added thereto while stirring and maintaining the whole under a nitrogen atmosphere. The xylene solution immediately turns dark colored, but upon crystallization of the sodium salt therefrom it becomes again light-colored. The reaction mixture is stirred for about 2 hours at 80°C until the development of ammonia has terminated. A solution of γ-dimethylaminopropyl chloride in toluene, prepared by setting free a corresponding amount of the free base from 17.4 parts of its hydrochloride salt by addition of aqueous sodium hydroxide solution in about 10% excess, extraction with toluene and drying for 2 hours over anhydrous sodium sulfate is added to the xylene solution containing the sodium salt mentioned above and the whole is stirred under reflux for 15 hours. Precipitated sodium chloride is filtered off and the filtrate is concentrated. The residue is diluted with ether, and the hydrochloride of 3- chloro-5-(γ-dimethylaminopropyl)-iminodibenzyl is precipitated by introducing dry, gaseous hydrogen chloride. It is filtered off under suction and purified by repeated recrystallization from acetone; the pure substance melts at 191.5°C to 192°C.
brand name
Anafranil (Tyco).
Therapeutic Function
Antidepressant
General Description
Clomipramine (Anafranil) is up to 50 times as potentas imipramine in some bioassays. This does not implyclinical superiority, but it might be informative about tricyclicand, possibly, other reuptake inhibitors. The chloro replacingthe H-substituent could increase potency by increasingdistribution to the CNS, but it is unlikely that this wouldgive the potency magnitude seen. It might be conjecturedthat an H-bond between the protonated amino group (as invivo) and the unshared electrons of the chloro substituent might stabilize a β-arylamine–like shape and give moreefficient competition for the transporter. The drug is anantidepressant. It is used in obsessive–compulsive disorder,an anxiety disorder that may have an element of depression.
Biological Activity
Potent, selective 5-HT uptake blocker. Antidepressant. Binds to the human 5-HT transporter with a K i of 0.05 nmol/l. Also available as part of the Serotonin Uptake Inhibitor Tocriset™ .
Veterinary Drugs and Treatments
In veterinary medicine, clomipramine hydrochloride is used primarily in dogs as a treatment for obsessive-compulsive disorders (ritualistic stereotypical behaviors) and may be useful for dominance aggression and anxiety (separation). Clomipramine hydrochloride may also be useful in cats, particularly for behaviors such as urine spraying. One prospective, double-blinded controlled study in cats with psychogenic alopecia comparing clomipramine (0.5 mg/kg PO daily) versus placebo showed no statistic differences in study parameters (Mertens, Torres et al. 2006).
Drug interactions
Potentially hazardous interactions with other drugs
Alcohol: increased sedative effect.
Analgesics: increased risk of CNS toxicity with
tramadol; possibly increased risk of side effects with
nefopam; possibly increased sedative effects with
opioids.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with amiodarone - avoid; increased
risk of ventricular arrhythmias with disopyramide,
flecainide or propafenone; avoid with dronedarone.
Antibacterials: increased risk of ventricular
arrhythmias with delamanid and moxifloxacin and
possibly telithromycin - avoid with delamanid and
moxifloxacin.
Anticoagulants: may alter anticoagulant effect of
coumarins.
Antidepressants: possibly increased serotonergic
effects with duloxetine; enhanced CNS excitation
and hypertension with MAOIs and moclobemide;
concentration possibly increased with SSRIs; risk
of ventricular arrhythmias with citalopram and
escitalopram - avoid; possible increased risk of
convulsions with vortioxetine.
Antiepileptics: convulsive threshold lowered;
concentration reduced by carbamazepine,
phenobarbital and possibly fosphenytoin, phenytoin
and primidone.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Antipsychotics: increased risk of ventricular
arrhythmias especially with droperidol, fluphenazine,
haloperidol, pimozide, sulpiride and zuclopenthixol
- avoid; increased risk of ventricular arrhythmias
with risperidone; increased antimuscarinic effects
with clozapine and phenothiazines; concentration
increased by antipsychotics.
Antivirals: increased risk of ventricular arrhythmias
with saquinavir - avoid; concentration possibly
increased with ritonavir.
Atomoxetine: increased risk of ventricular
arrhythmias and possibly convulsions.
Beta-blockers: increased risk of ventricular
arrhythmias with sotalol.
Clonidine: tricyclics antagonise hypotensive
effect; increased risk of hypertension on clonidine
withdrawal.
Cytotoxics: increased risk of ventricular arrhythmias
with arsenic trioxide.
Dapoxetine: possibly increased risk of serotonergic
effects - avoid.
Dopaminergics: avoid use with entacapone; CNS
toxicity reported with selegiline and rasagiline.
Methylthioninium: risk of CNS toxicity - avoid if
possible.
Metabolism
Clomipramine is extensively demethylated during
first-pass metabolism in the liver to its primary active
metabolite, desmethylclomipramine. Clomipramine has
been estimated to have a plasma elimination half-life of
about 21 hours; that of desmethylclomipramine is longer
(about 36 hours).
Paths of metabolism of both clomipramine and
desmethylclomipramine include hydroxylation and
N-oxidation. About two-thirds of a single dose of
clomipramine is excreted in the urine, mainly in the form
of its metabolites, either free or in conjugated form; the
remainder of the dose appears in the faeces.
Properties of Clomipramine hydrochloride
| Melting point: | 189-190°C |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | H2O: 25 mg/mL |
| form | powder |
| color | white to off-white |
| PH | 3.5~5.0(100g/l,25℃) |
| CAS DataBase Reference | 17321-77-6(CAS DataBase Reference) |
Safety information for Clomipramine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects H370:Specific target organ toxicity, single exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Clomipramine hydrochloride
| InChIKey | WIMWMKZEIBHDTH-UHFFFAOYSA-N |
Clomipramine hydrochloride manufacturer
Believe Pharma
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![3-Chloro-10,11-dihydro-5H-dibenzo[b,f]azepine](https://img.chemicalbook.in/CAS/GIF/32943-25-2.gif)
You may like
-
 Clomipramine hydrochloride 17321-77-6 98%View Details
Clomipramine hydrochloride 17321-77-6 98%View Details
17321-77-6 -
 Clomipramine CAS 17321-77-6View Details
Clomipramine CAS 17321-77-6View Details
17321-77-6 -
 CLOMIPRAMINE HYDROCHLORIDE CAS 17321-77-6View Details
CLOMIPRAMINE HYDROCHLORIDE CAS 17321-77-6View Details
17321-77-6 -
 Clomipramine HCL IP powderView Details
Clomipramine HCL IP powderView Details
17321-77-6 -
 Clomipramine Hydrochloride API PowderView Details
Clomipramine Hydrochloride API PowderView Details
17321-77-6 -
 Clomipramine Hydrochloride PowderView Details
Clomipramine Hydrochloride PowderView Details
17321-77-6 -
 Clomipramine Hydrochloride ApiView Details
Clomipramine Hydrochloride ApiView Details
17321-77-6 -
 Clomipramine HCLView Details
Clomipramine HCLView Details
17321-77-6
