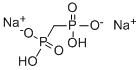
Clodronate disodium Salt
Synonym(s):Cl2MDP;Clodronate disodium;Clodronic acid disodium salt;DMDP
- CAS NO.:22560-50-5
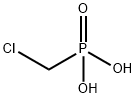
- Empirical Formula: CH5Cl2NaO6P2
- Molecular Weight: 268.88
- MDL number: MFCD01632786
- EINECS: 245-078-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
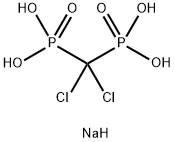
What is Clodronate disodium Salt?
Description
Clodronate disodium is a bone resorption inhibitor, useful as adjunct therapy in the treatment of osteolytic bone metastases and malignant hypercalcemia.
Chemical properties
White Solid
Originator
Proctor & Gamble (USA)
The Uses of Clodronate disodium Salt
A bisphosphonate antiresorptive agent; bone resorption inhibitor.
The Uses of Clodronate disodium Salt
Clodronic Acid Disodium Salt Hydrate is a bisphosphonate antiresorptive agent; bone resorption inhibitor.
What are the applications of Application
Clodronate, Disodium Salt is a bisphosphonate apoptosis inducing agent
Definition
ChEBI: The disodium salt of clodronic acid. It inhibits bone resorption and soft tissue calcification, and is used (generally as the tetrahydrate) as an adjunct in the treatment of severe hypercalcaemia associated with malignancy, and in the management of osteoly ic lesions and bone pain associated with skeletal metastases.
brand name
Bonefos (Leiras Oy, Finland).
Hazard
Moderately toxic by ingestion.
Biochem/physiol Actions
Analog of pyrophosphate ion that inhibits the osteoclastic activity leading to bone resorption and osteoporosis. The compound is used in cancer research, especially in skeletal metastases and breast carcinoma. When entrapped in liposomes, it is used for macrophage-selective depletion (macrophage "suicide" technique), especially in spleen and liver. Found also to inhibit collagenase and matrix metalloproteinase1.
Clinical Use
Bisphosphonate: Management of osteolytic lesions, hypercalcaemia and bone pain associated with skeletal metastases in patients with breast cancer or multiple myeloma
Safety Profile
A poison by intravenous route. Moderately toxic by ingestion, intraperitoneal routes. When heated to decomposition it emits toxic vapors of PO, and Cl-
Drug interactions
Potentially hazardous interactions with other drugs
Cytotoxics: concentration of estramustine increased.
Metabolism
Clodronate is not metabolised.
Over 70% of an intravenous dose is excreted unchanged
in the urine within 24 hours, the remainder being
sequestered to bone tissue. The substance which is bound
to bone is excreted more slowly, and the renal clearance is
about 75% of the plasma clearance.
Properties of Clodronate disodium Salt
| Melting point: | >330°C |
| storage temp. | room temp |
| solubility | Freely soluble in water, practically insoluble in ethanol (96 per cent), slightly soluble in methanol. |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 22560-50-5(CAS DataBase Reference) |
Safety information for Clodronate disodium Salt
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Clodronate disodium Salt
| InChIKey | HJKBJIYDJLVSAO-UHFFFAOYSA-L |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran



You may like
-
 Dichloromethylenediphosphonic acid disodium salt CAS 22560-50-5View Details
Dichloromethylenediphosphonic acid disodium salt CAS 22560-50-5View Details
22560-50-5 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
