Cinnamonitrile
Synonym(s):3-Phenylacrylonitrile
- CAS NO.:1885-38-7
- Empirical Formula: C9H7N
- Molecular Weight: 129.16
- MDL number: MFCD00001930
- EINECS: 217-552-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:53
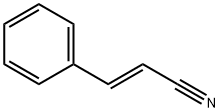
What is Cinnamonitrile?
Chemical properties
Clear colourless to yellow liquid, ractical}y insoluble in water, soluble in alcohol and oils.
Occurrence
Has apparently not been reported to occur in nature.
The Uses of Cinnamonitrile
Cinnamonitrile is a parent compound whose derivatives can be applied as corrosion inhibitors for API J55 steel.
The Uses of Cinnamonitrile
The parent compound whose derivatives can be applied as corrosion inhibitors for API J55 steel.
Preparation
From styryl bromide and potassium cyanide (Arctander, 1969).
Synthesis Reference(s)
Journal of the American Chemical Society, 81, p. 6340, 1959 DOI: 10.1021/ja01532a069
Tetrahedron Letters, 21, p. 2161, 1980 DOI: 10.1016/S0040-4039(00)78986-6
Toxicity evaluation
The acute oral LD50 value in rats was reported as 4.15g/kg (3.67-4.63 g/kg) and the acute dermal LD50 value in rabbits exceeded 5 g/kg (Moreno, 1974). In rabbits, iv injection of cinnamyl nitrile (20.3 mg/kg) caused death in 10 min. In guinea-pigs and rabbits, cinnamyl nitrile was found to produce the same effects as hydrogen cyanide, with almost equal toxicity, and rabbits given doses higher than the lethal dose were found to die almost as rapidly as those given hydrogen cyanide (Hunt, 1923).
Flammability and Explosibility
Not classified
Metabolism
The toxicity of organic cyanides appears to depend almost entirely on whether they can be metabolized in the body to the free cyanide ion, and on the rate and extent of this conversion and the rate of detoxication of cyanide to thiocyanate. In general, alkyl and arylalkyl cyanides are metabolized with the formation of hydrogen cyanide, while the nitrile group of aryl cyanides is relatively stable. The high toxicity of iv-administered cinnamyl nitrile and the rapidity of death suggest that in rabbits this compound is metabolically converted to hydrogen cyanide (Hunt, 1923; Williams, 1959).
Properties of Cinnamonitrile
| Melting point: | 18-20 °C (lit.) |
| Boiling point: | 254-255 °C (lit.) |
| Density | 1.028 g/mL at 25 °C (lit.) |
| vapor pressure | 1.2Pa at 20℃ |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | soluble in Alcohol |
| form | Liquid |
| color | Clear colorless to yellow |
| Odor | at 10.00 % in dipropylene glycol. nitrile cinnamon cassia deep cumin |
| Water Solubility | insoluble |
| Sensitive | Air & Light Sensitive |
| BRN | 1209546 |
| CAS DataBase Reference | 1885-38-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propenenitrile, 3-phenyl-, (E)-(1885-38-7) |
| EPA Substance Registry System | 2-Propenenitrile, 3-phenyl-, (2E)- (1885-38-7) |
Safety information for Cinnamonitrile
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Cinnamonitrile
| InChIKey | ZWKNLRXFUTWSOY-QPJJXVBHSA-N |
Cinnamonitrile manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 1885-38-7 Cinnamonitrile, 97% 99%View Details
1885-38-7 Cinnamonitrile, 97% 99%View Details
1885-38-7 -
 Cinnamonitrile extrapure CAS 1885-38-7View Details
Cinnamonitrile extrapure CAS 1885-38-7View Details
1885-38-7 -
 Cinnamonitrile CAS 1885-38-7View Details
Cinnamonitrile CAS 1885-38-7View Details
1885-38-7 -
 Cinnamonitrile, 97% CAS 1885-38-7View Details
Cinnamonitrile, 97% CAS 1885-38-7View Details
1885-38-7 -
 Cinnamonitrile CAS 1885-38-7View Details
Cinnamonitrile CAS 1885-38-7View Details
1885-38-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
