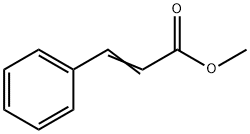
Cinnamaldehyde
Synonym(s):3-Phenylprop-2-enal;Cinnamal;Cinnamic aldehyde
- CAS NO.:104-55-2
- Empirical Formula: C9H8O
- Molecular Weight: 132.16
- MDL number: MFCD00007000
- EINECS: 203-213-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
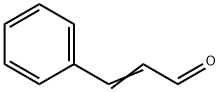
What is Cinnamaldehyde?
Description
Cinnamic aldehyde is used as a flavoring agent, ingredient of fragrance in soft drinks, ice creams, dentifrices, pastries, chewing-gum, etc. It can induce both contact urticaria and delayed-type reactions. It can be implicated in contact dermatitis in those who work in the perfume industry or food handlers. Cinnamic aldehyde is contained in the "fragrance mix".
Chemical properties
Cinnamaldehyde exists as yellowish to greenish-yellow oily liquid with a Strong pungent, spicy, cinnamon odor. It is normally insoluble in water and many organic solvents but is miscible with alcohol and other flavoring oils. Exposure to air will result in thickening and oxidation.

Cinnamaldehyde (cinnamic aldehyde, benzylideneactaldehyde, phenylacrolein, 3-phenylpropenal, 3-phenyl-2-propenal) is a saturated aldehyde with an aromatic ring that is a yellowish, oily liquid at room temperature. It is used as a flavoring and aromatic agent in a variety of food products, perfumes, and household products. It has also seen use as a rubber reinforcing agent. A burning taste that produces the odor and flavor of the spice may be found with this aromatic aldehyde. Cinnamaldehyde has also been used as an attractant for insect control, in the preparation of corrosion inhibitors, and as a coating for metals. Although extensively used in industry, it is also a natural constituent of cinnamon leaves and bark, some essential oils, and other plant products.
Occurrence
Reported found in celery seed, cinnamon, cinnamon leaf, cassia leaf, clove stem and lemon balm.
The Uses of Cinnamaldehyde
Cinnamaldehyde is used in flavor and perfumes.It occurs in cinnamon oils.
The Uses of Cinnamaldehyde
In the flavor and perfume industry.
The Uses of Cinnamaldehyde
Cinnamic aldehyde is a common ingredient in perfumes for household products like deodorizers, detergents, and soap; flavor in toothpaste, sweets, ice cream, soft drinks, chewing gums, and cakes; in balsam of Tolu and Peru, hyacinth plant, spices, cinnamon, Ceylon and cassia oil; some perfumery uses (Canoe; hyacinth; bubblegum; Balsam; Cassia); natural occurrence (cinnamon).
Definition
ChEBI: (E)-cinnamaldehyde is the E (trans) stereoisomer of cinnamaldehyde, the parent of the class of cinnamaldehydes. It has a role as a hypoglycemic agent, an EC 4.3.1.24 (phenylalanine ammonia-lyase) inhibitor, a vasodilator agent, an antifungal agent, a flavouring agent, a plant metabolite and a sensitiser. It is a 3-phenylprop-2-enal and a member of cinnamaldehydes.
Preparation
Cinnamaldehyde is industrially prepared by condensation of benzaldehyde and acetaldehyde in dilute alkali solution.
Aroma threshold values
Detection at 50 to 750 ppb.
Taste threshold values
Taste characteristics at 0.5 ppm: spicy, cinnamon and cinnamon bark.
General Description
Yellow oily liquid with a cinnamon odor and sweet taste.
Air & Water Reactions
Thickens on exposure to air. May be unstable to prolonged exposure to air. Slightly water soluble .
Reactivity Profile
Cinnamaldehyde reacts with sodium hydroxide owing to aerobic oxidation.
Health Hazard
Cinnamaldehyde can cause moderate to severeskin irritation. Exposure to 40 mg in48 hours produced a severe irritation effecton human skin. The toxicity of this compoundwas low to moderate on test subjects,depending on the species and the toxicroutes. However, when given by oral routein large amounts, its poisoning effect wassevere. Amounts greater than 1500 mg/kghave produced a wide range of toxic effectsin rats, mice, and guinea pigs. The symptomswere respiratory stimulation, somnolence,convulsion, ataxia, coma, hypermotility, anddiarrhea.
LD50 value, oral (guinea pigs): 1150 mg/kg
Cinnamaldehyde is a mutagen. Its carcinogeniceffect is not established.
Fire Hazard
Cinnamaldehyde is combustible.
Agricultural Uses
Fungicide, Insecticide: Used as an antifungal agent, corn rootworm attractant, and dog and cat repellent. Can be used on soil casing for mushrooms, row crops, turf and all food commodities. Not listed for use in EU countries.
Trade name
ADIOS®; ZIMTALDEHYDE®; ZIMTALDEHYDE® LIGHT
Contact allergens
This perfumed molecule is used as a fragrance in perfumes, a flavoring agent in soft drinks, ice creams, dentifrices, pastries, chewing-gum, etc. It can induce both contact urticaria and delayed-type reactions. It can be responsible for dermatitis in the perfume industry or in food handlers. Cinnamic aldehyde is contained in “fragrance mix.” As a fragrance allergen, it has to be mentioned by name in cosmetics within the EU.
Anticancer Research
This is promising in antitumor activity against NSCLC cells. The cells were inducedin apoptosis and also the epithelial-mesenchymal transition was reversed affectingthe Wnt/b-catenin pathway (Bouyahya et al. 2016).
Safety Profile
Poison by intravenous and parenteral routes. Moderately toxic by ingestion and intraperitoneal routes. A severe human skin irritant. Mutation data reported. Combustible liquid. May ipte after a delay period in contact with NaOH. When heated to decomposition it emits acrid smoke and fumes. See also ALDEHYDES.
Synthesis
By isolation from natural sources; synthetically, by condensation of benzaldehyde with acetaldehyde in the presence of sodium or calcium hydroxide.
Potential Exposure
Botanical fungicide and insecticide. Used as an antifungal agent, corn rootworm attractant, and dog and cat repellent. Can be used on soil casing for mushrooms, row crops, turf, and all food commodities. Not listed for use in EU countries.
Toxicity
Cinnamaldehyde is used in agriculture because of its low toxicity, but it is a skin irritant.Cinnamaldehyde may cause allergic contact stomatitis in sensitised individuals, however allergy to the compound is believed to be uncommon.
Shipping
UN1989 Aldehydes, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid
Incompatibilities
Aldehydes are frequently involved in selfcondensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, ketones, azo dyes, caustics, boranes, hydrazines
Waste Disposal
Incineration. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers.
Properties of Cinnamaldehyde
| Melting point: | −9-−4 °C(lit.) |
| Boiling point: | 248 °C (lit.) |
| Density | 1.05 g/mL at 25 °C (lit.) |
| vapor density | 4.6 (vs air) |
| vapor pressure | <0.1 hPa (20 °C) |
| FEMA | 2286 | CINNAMALDEHYDE |
| refractive index | n |
| Flash point: | 160 °F |
| storage temp. | Store below +30°C. |
| solubility | 1g/l soluble |
| form | Liquid |
| pka | 0[at 20 ℃] |
| Specific Gravity | 1.05 |
| color | Clear yellow |
| Odor | Strong odor of cinnamon |
| Water Solubility | Slightly soluble |
| Merck | 13,2319 |
| JECFA Number | 656 |
| Dielectric constant | 16.9(24℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases. |
| CAS DataBase Reference | 104-55-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Cinnamylaldehyde(104-55-2) |
| EPA Substance Registry System | Cinnamaldehyde (104-55-2) |
Safety information for Cinnamaldehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cinnamaldehyde
| InChIKey | KJPRLNWUNMBNBZ-QPJJXVBHSA-N |
Cinnamaldehyde manufacturer
JSK Chemicals
Prakash Chemicals International Private Limited
M. P. Aromas
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
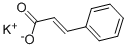
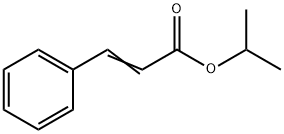
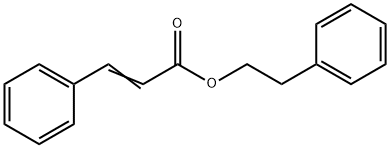
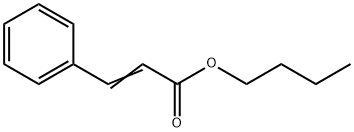
You may like
-
 Cinnamaldehyde SUPPLIERView Details
Cinnamaldehyde SUPPLIERView Details
104-55-2 -
 CINNAMIC ALDEHYDE 99%View Details
CINNAMIC ALDEHYDE 99%View Details -
 Cinnamic Aldehyde 99%View Details
Cinnamic Aldehyde 99%View Details -
 Cinnamaldehyde 99%View Details
Cinnamaldehyde 99%View Details -
 Cinnamic Aldehyde (C6H5CH), 99%, 220 Kg DrumView Details
Cinnamic Aldehyde (C6H5CH), 99%, 220 Kg DrumView Details
104-55-2 -
 Cinnamic Aldehyde,C9H8O,CAS 104-55-2, 5,35,220kgs Drum, For Industrial UseView Details
Cinnamic Aldehyde,C9H8O,CAS 104-55-2, 5,35,220kgs Drum, For Industrial UseView Details
104-55-2 -
 Cinnamic AldehydeView Details
Cinnamic AldehydeView Details
104-55-2 -
 Cinnamic Aldehyde Aromatic Chemical, 100%, 50 L DrumView Details
Cinnamic Aldehyde Aromatic Chemical, 100%, 50 L DrumView Details
104-55-2
