Cinnamyl alcohol
Synonym(s):3-Phenyl-2-propen-1-ol
- CAS NO.:104-54-1
- Empirical Formula: C9H10O
- Molecular Weight: 134.18
- MDL number: MFCD00002921
- EINECS: 203-212-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
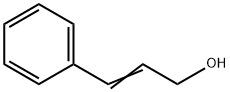
What is Cinnamyl alcohol?
Description
Occupational cases of contact dermatitis were reported in the perfurne industry. Patch tests can also be positive in food handlers. Cinnamic alcohol is contained in the "fragrance mix".
Chemical properties
Cinnamyl alcohol can exist in (Z)-[4510-34-3] and (E)-[4407-36-7] forms. Although both isomers occur in nature, the (E)-isomer is far more abundant and is present, for example, in styrax oil. (E)-Cinnamyl alcohol is a colorless, crystalline solid with a hyacinth-like balsamic odor.
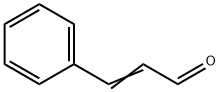
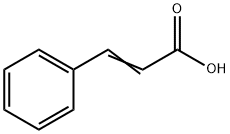
Cinnamyl alcohol can be dehydrogenated to give cinnamaldehyde and oxidized to give cinnamic acid. Hydrogenation yields 3-phenylpropanol and/or 3-cyclohexylpropanol. Reaction with carboxylic acids or carboxylic acid derivatives results in the formation of cinnamyl esters, some of which are used as fragrance materials.
Chemical properties
Cinnamyl alcohol has a pleasant, floral odor and bitter taste.
Occurrence
Occurring as an ester or in the free state in hyacinth, Aristolochia clematis, Xanthorrhoea hastilis and in the essence of daffodil flowers. It is also reported found in guava fruit and peel, lemon peel oil, cassia leaf, Bourbon vanilla and cinnamon bark, leaf and root.
The Uses of Cinnamyl alcohol
Cinnamyl alcohol was used to study the alkylation of 2,4-di-tert-butylphenol by cinnamyl alcohol using aluminum-containing mesoporous ethane-silica catalyst. It was used to study gold nanoparticles supported on titanium dioxide catalysed oxidative coupling of alcohols and amines to form the corresponding imines.
What are the applications of Application
Cinnamyl alcohol is mainly used in the following fields:
(1) Used as a flavouring or flavouring agent in cosmetics;
(2) Used as a deodorant in perfumes;
(3) Intermediates in organic synthesis;
(4) Allergic epidermal patch test.
Definition
ChEBI: A primary alcohol comprising an allyl core with a hydroxy substituent at the 1-position and a phenyl substituent at the 3-position (geometry of the C2C bond unspecified).
Preparation
By reduction of cinnamic aldehyde.
Aroma threshold values
Detection: 1 ppm; cis- form, 81 ppb; trans- form, 2.8 ppm
Taste threshold values
Taste characteristics at 20 ppm: green, floral, spicy and honey with a fermented yeasty nuance.
Synthesis Reference(s)
Chemistry Letters, 5, p. 581, 1976
The Journal of Organic Chemistry, 59, p. 6378, 1994 DOI: 10.1021/jo00100a046
Tetrahedron Letters, 34, p. 257, 1993 DOI: 10.1016/S0040-4039(00)60561-0
Flammability and Explosibility
Not classified
Contact allergens
Cinnamyl alcohol occurs (in esterified form) in storax, Myroxylon pereirae, cinnamon leaves, and hyacinth oil. It is obtained by the alkaline hydrolysis of storax and prepared synthetically by reducing cinnamal diacetate with iron filings and acetic acid, and from cinnamaldehyde by Meerwein-Ponndorf reduction with aluminum isopropoxide. Cinnamyl alcohol is contained in the “fragrance mix.” As a fragrance allergen, it has to be mentioned by name in cosmetics within the EU. Occupational cases of contact dermatitis were reported in perfume industry. Patch tests can be positive in food handlers.
Synthesis
Obtained originally by saponification of extraction from storax; synthetically, by reduction of cinnamaldehyde with sodium or potassium hydroxide.
Purification Methods
Crystallise the alcohol from diethyl ether/pentane. [Beilstein 6 I 281.]
Hazard
Cinnamyl alcohol is potentially skin sensitising and may cause symptoms such as redness, bumpiness or itching of the skin. A patch test screening several groups of perfumed materials was performed on 20 subjects sensitised to the perfume. The most common allergen was cinnamyl alcohol (15 out of 20).
Properties of Cinnamyl alcohol
| Melting point: | 30-33 °C (lit.) |
| Boiling point: | 250 °C (lit.) |
| Density | 1.044 g/mL at 25 °C (lit.) |
| vapor density | 4.6 (vs air) |
| vapor pressure | <0.01 mm Hg ( 25 °C) |
| refractive index | 1.5819 |
| FEMA | 2294 | CINNAMYL ALCOHOL |
| Flash point: | >230 °F |
| storage temp. | -20°C |
| solubility | H2O: soluble |
| form | Fused Low Melting Crystalline Solid |
| pka | 0.852[at 20 ℃] |
| color | White |
| Specific Gravity | 1.044 |
| Odor | at 100.00 %. sweet balsam hyacinth spicy green powdery cinnamic |
| Water Solubility | 1.8 g/L (20 ºC) |
| Merck | 14,2302 |
| JECFA Number | 647 |
| BRN | 1903999 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 104-54-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propen-1-ol, 3-phenyl-(104-54-1) |
| EPA Substance Registry System | 3-Phenyl-2-propen-1-ol (104-54-1) |
Safety information for Cinnamyl alcohol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Cinnamyl alcohol
| InChIKey | OOCCDEMITAIZTP-QPJJXVBHSA-N |
Cinnamyl alcohol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![(aR)-alpha-[(1S)-1-[2,6-Dimethoxy-4-(2-propen-1-yl)phenoxy]ethyl]-4-hydroxy-3-methoxybenzenemethanol](https://img.chemicalbook.in/CAS/GIF/171485-39-5.gif)
You may like
-
 CINNAMIC ALCOHOL 99%View Details
CINNAMIC ALCOHOL 99%View Details -
 Cinnamyl alcohol 95% CAS 104-54-1View Details
Cinnamyl alcohol 95% CAS 104-54-1View Details
104-54-1 -
 Cinnamic Alcohol CAS Number: 104-54-1 Chemical formula: C9HView Details
Cinnamic Alcohol CAS Number: 104-54-1 Chemical formula: C9HView Details
104-54-1 -
 Cinnamyl AlcoholView Details
Cinnamyl AlcoholView Details
104-54-1 -
 Cinnamyl Alcohol CAS 104-54-1View Details
Cinnamyl Alcohol CAS 104-54-1View Details
104-54-1 -
 Cinnamic AlcoholView Details
Cinnamic AlcoholView Details
104-54-1 -
 Cinnamic AlcoholView Details
Cinnamic AlcoholView Details
104-54-1 -
 Cinnamyl alcoholView Details
Cinnamyl alcoholView Details
104-54-1
