Trimethoxysilane
- CAS NO.:2487-90-3
- Empirical Formula: C3H10O3Si
- Molecular Weight: 122.2
- MDL number: MFCD00008340
- EINECS: 219-637-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
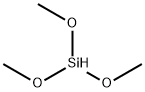
What is Trimethoxysilane?
Chemical properties
Colorless clear liquid
The Uses of Trimethoxysilane
Trimethoxysilane is used as a catalyst in the hydrogenation of furfural to furfuryl alcohol.
General Description
A colorless liquid. Slightly soluble in water and denser than water. Hence floats on water. Very toxic by ingestion, inhalation or skin absorption. May also be corrosive to skin and eyes.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Trimethoxysilane may accumulate static electricity; hazardous polymerization may occur.
Health Hazard
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Flammable
Safety Profile
Moderately toxic by inhalation. Mildly toxic by ingestion and skin contact. When heated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
Likely impurities are Si(OMe)4 and H2Si(OMe)2. Efficient fractionation is essential for removing these impurities [IR: Sternbach & MacDiarmid J Am Chem Soc 81 5109 1959, Heilfrich & Hausen Chem Ber 57 795 1924, Beilstein 1 IV 1266.]
Properties of Trimethoxysilane
| Melting point: | −115 °C(lit.) |
| Boiling point: | 81 °C(lit.) |
| Density | 0.96 g/mL at 25 °C(lit.) |
| vapor density | >1 (vs air) |
| vapor pressure | <7.2 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 24 °F |
| storage temp. | Flammables area |
| solubility | Chloroform (Sparingly), Methanol (Sparingly) |
| form | liquid |
| Specific Gravity | 0.860 |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| BRN | 1697990 |
| Stability: | May decompose upon exposure to air or water. Incompatible with strong oxidizing agents, strong bases, strong acids. |
| CAS DataBase Reference | 2487-90-3(CAS DataBase Reference) |
| EPA Substance Registry System | Trimethoxysilane (2487-90-3) |
Safety information for Trimethoxysilane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trimethoxysilane
| InChIKey | YUYCVXFAYWRXLS-UHFFFAOYSA-N |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


You may like
-
 2487-90-3 Trimethoxysilane 98%View Details
2487-90-3 Trimethoxysilane 98%View Details
2487-90-3 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
