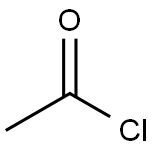
Chloroacetyl chloride
Synonym(s):Chloroacetyl chloride
- CAS NO.:79-04-9
- Empirical Formula: C2H2Cl2O
- Molecular Weight: 112.94
- MDL number: MFCD00000725
- EINECS: 201-171-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
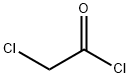
What is Chloroacetyl chloride?
Description
Chloroacetyl chloride is a colorless to yellowish liquid with a very pungent, extremely irritating, odor.Molecular weight=112.95; Specific gravity (H2O:1) 51.42; Boiling point=105℃; Freezing/Meltingpoint=222℃; Vapor pressure=19 mmHg at 20℃.NFPA 704 M Rating System: Health 3, Flammability 1,Reactivity 1 . Reacts violently with water.
Chemical properties
Chloroacetyl chloride is a colorless to yellowish liquid with a very pungent, extremely irritating, odor.
The Uses of Chloroacetyl chloride
Chloroacetyl chloride is an intermediate in the production of alachlor and butachlor. It is used to prepare phenacyl chloride through Friedel-Crafts acylation of benzene.
The Uses of Chloroacetyl chloride
In the synthesis of organic compounds.
The Uses of Chloroacetyl chloride
Intermediate in manufacture of chloroacetophenone and various other chemicals
Definition
Chloroacetyl chloride is classified as an acyl halide. It is a colorless to light yellow liquid with a strong, pungent odor. This compound is highly toxic when inhaled and can corrode both metal surfaces and tissues.
What are the applications of Application
Chloroacetyl chloride can be used for chloroacetylation and chemical modification of poly(glycidyl methacrylate).
Chloroacetyl chloride was used to study the in vitro metabolism of chloroacetamide herbicides by rat and human liver microsomes. Chloroacetyl chloride was used in the synthesis of some novel quinoline derivatives having antileishmanial activity.
Preparation
Chloroacetyl chloride is usually manufactured from chloroacetic acid by reaction with phosphorus trichloride, thionyl chloride, sulfuryl chloride, or phosgene. It is also obtained by chlorination of acetyl chloride in the presence of stronger aliphatic acids, preferably chloroacetic acids, or from sodium chloroacetate and the usual chlorinating agents.
Koenig G et al; Chloroacetic Acids. Ullmann's Encyclopedia of Industrial Chemistry 7th ed. (1999-2018). NY, NY: John Wiley & Sons. Online Posting Date: October 15, 2012
General Description
A colorless to light yellow liquid with a very pungent odor. Very toxic by inhalation. Corrosive to metals and tissue.
Reactivity Profile
Chloroacetyl chloride reacts rapidly with water. Incompatible with strong oxidizing agents, alcohols, bases (including amines). May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Hazard
Irritant to eyes, corrosive to skin. Upper respiratory tract irritant.
Health Hazard
Inhalation causes severe irritation of upper respiratory system. External contact causes severe irritation of eyes and skin. Ingestion causes severe irritation of mouth and stomach.
Safety Profile
Poison by ingestion and intravenous routes. Mildly toxic by inhalation. Corrosive. A lachrymator. When heated to decomposition it emits toxic fumes of Cl-.
Potential Exposure
Highly toxic by inhalation. Chloroacetyl chloride is used in the manufacture of acetophenone. It is used in the manufacture of a number of pesticides including: alachlor, allidochlor, butachlor, dimethachlor, formothion, mecarbam, metolachlor, propachlor. It is also used in the manufacture of pharmaceuticals, such as chlordiazepoxide hydrochloride, diazepam, lidocaine, mianserin.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24-48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray.
Carcinogenicity
Chloroacetyl chloride was not genotoxic in a number of assays.
storage
Chloroacetyl chloride must carry a“CORROSIVE” label. It falls in Hazard Class 8 andPacking Group I.
Shipping
UN1752 Chloroacetyl chloride, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 8-Corrosive material, Inhalation Hazard Zone B.
Incompatibilities
Reacts violently with water, steamforming chloroacetic acid and hydrogen chloride gas. Reacts with alcohols, powdered metals; sodium amide; combustibles; and many organics, causing toxic fumes, fire, and explosion hazard. On contact with air it emits corrosive gas. Decomposes when heated forming phosgene gas. Corrosive to metals.
Waste Disposal
It may be discharged into sodium bicarbonate solution, then flushed to the sewer with water.
Properties of Chloroacetyl chloride
| Melting point: | −22 °C(lit.) |
| Boiling point: | 105-106 °C(lit.) |
| Density | 1.419 g/mL at 20 °C |
| vapor density | 3.9 (vs air) |
| vapor pressure | 60 mm Hg ( 41.5 °C) |
| refractive index | n |
| Flash point: | >100°C |
| storage temp. | Store at RT. |
| solubility | Miscible with ethyl ether, acetone, benzene and carbon tetrachloride. |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| Odor | strong pungent odor |
| Water Solubility | reacts |
| FreezingPoint | -22.5℃ |
| Sensitive | Moisture Sensitive |
| Merck | 14,2067 |
| BRN | 605439 |
| Exposure limits | ACGIH: TWA 0.05 ppm; STEL 0.15 ppm (Skin) NIOSH: IDLH 1.3 ppm; TWA 0.05 ppm(0.2 mg/m3) |
| Stability: | Stable. Incompatible with strong bases, alcohols, strong oxidizing agents. May react violently on exposure to water or moisture. |
| CAS DataBase Reference | 79-04-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetyl chloride, chloro-(79-04-9) |
| EPA Substance Registry System | Chloroacetyl chloride (79-04-9) |
Safety information for Chloroacetyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H372:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Chloroacetyl chloride
Chloroacetyl chloride manufacturer
JSK Chemicals
TRUUCHEM TECHNOLOGIES PRIVATE LIMITED
CEFA CILINAS BIOTICS PVT LTD
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran


![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)

You may like
-
 Chloroacetyl chloride 98%View Details
Chloroacetyl chloride 98%View Details -
 79-04-9 98%View Details
79-04-9 98%View Details
79-04-9 -
 Chloroacetyl chloride CAS 79-04-9View Details
Chloroacetyl chloride CAS 79-04-9View Details
79-04-9 -
 Chloroacetyl chloride CAS 79-04-9View Details
Chloroacetyl chloride CAS 79-04-9View Details
79-04-9 -
 Chloroacetyl chloride CAS 79-04-9View Details
Chloroacetyl chloride CAS 79-04-9View Details
79-04-9 -
 Chloroacetyl chloride 98%View Details
Chloroacetyl chloride 98%View Details
79-04-9 -
 CHLORO ACETYL CHLORIDE CASView Details
CHLORO ACETYL CHLORIDE CASView Details -
 CHLOROACETYL CHLORIDE For Synthesis CAS 79-04-9View Details
CHLOROACETYL CHLORIDE For Synthesis CAS 79-04-9View Details
79-04-9
