Dichloroacetyl chloride
- CAS NO.:79-36-7
- Empirical Formula: C2HCl3O
- Molecular Weight: 147.39
- MDL number: MFCD00000840
- EINECS: 201-199-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
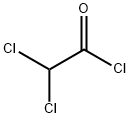
What is Dichloroacetyl chloride?
Chemical properties
Dichloroacetyl chloride (DCAC) is a colourless to light yellow fuming liquid with an acrid, penetrating odor. It is irritating to the eyes and mucous membranes (HSDB 2003b). DCAC is insoluble in water, but decomposes quickly to form HCl and dichloroacetic acid (t1/2 of hydrolysis of 0.0023 seconds in water at 25°C, and 0.2 seconds in 89.1:10.9 water-acetone at -20°C (Prager et al. 2001, Ugi and Beck 1961).
The Uses of Dichloroacetyl chloride
Dichloroacetyl Chloride is a reagent used in the synthesis of substituted benzothiazoles exhibiting antitumor and anticancer activity.
Definition
ChEBI: Dichloroacetyl chloride is the acyl chloride obtained by displacement of the hydroxy group of dichloroacetic acid by chloride. It has a role as a hapten. It derives from a dichloroacetic acid.
Preparation
Dichloroacetyl chloride can be synthesized by the reaction of dichloroacetic acid and chlorosulfonic acid. Or from the oxidation of trichloroethylene. Trichloroethylene and azobisisobutyronitrile (catalyst) were heated to 100°C, and oxygen was introduced to react under 0.6MPa pressure, maintaining the oil bath temperature at 110°C, and reacted for 10h, The product dichloroacetyl chloride is distilled off under normal pressure.
General Description
Dichloroacetyl chloride is a colorless liquid with a pungent odor. Flash point 151°F Boiling point 107-108°F. Vapors are irritating to the eyes and mucous membranes. Corrosive to metals and tissue.
Air & Water Reactions
Fumes in air. Decomposed by water to dichloroacetic acid and hydrochloric acid, both corrosive, with release of heat. (NIP).
Reactivity Profile
Solutions in acetone are stable for less than two hours and fresh solution should be prepared before each use (NIP). May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
Questionable carcinogen with experimental tumorigenic data. Moderately toxic by ingestion, inhalation, and skin contact. Corrosive to the skin, eyes, and mucous membranes. Combustible when exposed to heat or flame. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORIDES.
Properties of Dichloroacetyl chloride
| Melting point: | <25 °C |
| Boiling point: | 107-108 °C (lit.) |
| Density | 1.532 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 66 °C |
| storage temp. | 2-8°C |
| solubility | Chloroform, Hexanes |
| form | Liquid |
| color | Clear colorless to light yellow |
| Specific Gravity | 1.537 (20/4℃) |
| Water Solubility | MAY DECOMPOSE |
| Sensitive | Moisture Sensitive |
| Merck | 14,3053 |
| BRN | 1209426 |
| Stability: | Stable. Combustible. Incompatible with water, alcohols and oxidizing agents. Fumes in air. |
| CAS DataBase Reference | 79-36-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetyl chloride, dichloro-(79-36-7) |
| EPA Substance Registry System | Dichloroacetyl chloride (79-36-7) |
Safety information for Dichloroacetyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H350:Carcinogenicity H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Dichloroacetyl chloride
| InChIKey | FBCCMZVIWNDFMO-UHFFFAOYSA-N |
Dichloroacetyl chloride manufacturer
JSK Chemicals
ASM Organics
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


You may like
-
 Dichloroacetyl chloride, 97% CAS 79-36-7View Details
Dichloroacetyl chloride, 97% CAS 79-36-7View Details
79-36-7 -
 Dichloroacetyl chloride CAS 79-36-7View Details
Dichloroacetyl chloride CAS 79-36-7View Details
79-36-7 -
 Dichloroacetyl chloride 96% CAS 79-36-7View Details
Dichloroacetyl chloride 96% CAS 79-36-7View Details
79-36-7 -
 Dichloroacetyl Chloride CAS 79-36-7View Details
Dichloroacetyl Chloride CAS 79-36-7View Details
79-36-7 -
 Dichloroacetyl chloride CAS 79-36-7View Details
Dichloroacetyl chloride CAS 79-36-7View Details
79-36-7 -
 79-36-7 98%View Details
79-36-7 98%View Details
79-36-7 -
 Dichloroacetyl chloride, 98% 99%View Details
Dichloroacetyl chloride, 98% 99%View Details
79-36-7 -
 609-15-4View Details
609-15-4View Details
609-15-4
