Chloroacetic anhydride
Synonym(s):Monochloroacetic acid anhydride
- CAS NO.:541-88-8
- Empirical Formula: C4H4Cl2O3
- Molecular Weight: 170.97
- MDL number: MFCD00000929
- EINECS: 208-794-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
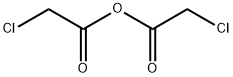
What is Chloroacetic anhydride?
Chemical properties
brownish semi-transparent crystals, chunks or low
The Uses of Chloroacetic anhydride
In N-acetylation of amino acids in alkaline solution; preparation of cellulose chloroacetates.
The Uses of Chloroacetic anhydride
Chloroacetic anhydride has been used in the synthesis of 3,3-bis(sulfonato)-4,4-bis(chloroacetamido)azobenzene (BSBCA), a water-soluble, thiol-reactive and photo-switchable cross-linker, D,L-7-azatryptophan, 2-methyl-[3,4-di-O-acetyl-6-O-(chloroacetyl)-1,2-dideoxy-α-D-glucopyrano]-[2,1:4,5]-2-oxazoline.
Preparation
chloroacetic anhydride Synthesis: Powdered s odium monochloroacetate(113.3g, 1.138 mol) was added slowly for a period of 15 min at room temperature (which slowly raises to 60°C) to a stirred solution of commercially available chloroaceyl chloride (127.5g, 1mol) in dry benzene (136mL) was refluxed for 9h. Salts were filtered off, the filtrate was cooled to room temperature, diluted with n-hexane and further cooled to 0°C. The solid separated was then filtered, washed with dry hexane and dried to yield chloroacetic anhydride as white crystalline compound in 60% yield (80g); mp=46-49°C [Lit. mp=46°C]. Alternatively, the benzene layer was directly subjected to high vacuum distillation.The product collected at 109-10°C at 10mm vacuum on cooling chloroacetic anhydride in lump form in 70 % yield (93g). It was observed that, the latter method gives anhydride in lump form while the former method gives in crystalline form.
Purification Methods
Crystallise the it from *benzene. [Eglinton et al. J Chem Soc 1860 1954, Beilstein 2 IV 487.]
Properties of Chloroacetic anhydride
| Melting point: | 51 °C |
| Boiling point: | 203 °C |
| Density | 1.449 |
| vapor pressure | <0.75 mm Hg ( 20 °C) |
| refractive index | 1.4730 (estimate) |
| Flash point: | >230 °F |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | Freely soluble in ether, chloroform. Slightly soluble in benzene. Insoluble in cold ligroin |
| form | Crystals, Chunks or Low Melting Mass |
| color | brown |
| Sensitive | Moisture Sensitive |
| Merck | 14,2113 |
| BRN | 774533 |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 541-88-8(CAS DataBase Reference) |
| EPA Substance Registry System | Acetic acid, chloro-, anhydride (541-88-8) |
Safety information for Chloroacetic anhydride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Chloroacetic anhydride
| InChIKey | PNVPNXKRAUBJGW-UHFFFAOYSA-N |
Chloroacetic anhydride manufacturer
Aizant Drug Research Solutions Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![N-[[2-[[[4-(Aminoiminomethyl)phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-(2-pyridinyl)-beta-alanine ethyl ester hydrochloride](https://img.chemicalbook.in/CAS2/GIF/211914-50-0.gif)
![3-[(3-AMINO-4-METHYLAMINO-BENZOYL)-PYRIDIN-2-YL-AMINO]-PROPIONIC ACID ETHYL ESTER](https://img.chemicalbook.in/CAS/GIF/212322-56-0.gif)

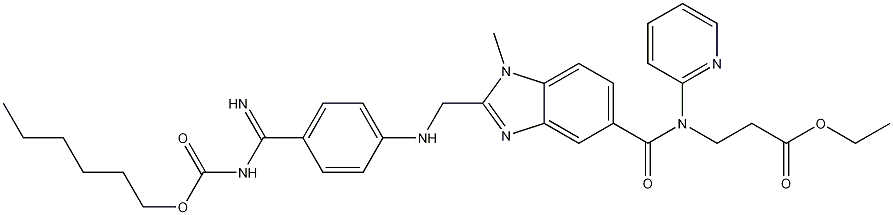
![3-[[[2-[[(4-Cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]pyridin-2-ylamino]propionic acid ethyl ester](https://img.chemicalbook.in/CAS2/GIF/211915-84-3.gif)
You may like
-
 541-88-8 Chloroacetic anhydride 98%View Details
541-88-8 Chloroacetic anhydride 98%View Details
541-88-8 -
 Chloroacetic anhydride 95% CAS 541-88-8View Details
Chloroacetic anhydride 95% CAS 541-88-8View Details
541-88-8 -
 Chloroacetic anhydride 97.00% CAS 541-88-8View Details
Chloroacetic anhydride 97.00% CAS 541-88-8View Details
541-88-8 -
 Chloroacetic Anhydride CAS 541-88-8View Details
Chloroacetic Anhydride CAS 541-88-8View Details
541-88-8 -
 Chloroacetic Anhydride CAS 541-88-8View Details
Chloroacetic Anhydride CAS 541-88-8View Details
541-88-8 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
