Phenylacetaldehyde
Synonym(s):α-Tolyaldehyde;2-Phenylacetaldehyde
- CAS NO.:122-78-1
- Empirical Formula: C8H8O
- Molecular Weight: 120.15
- MDL number: MFCD00006993
- EINECS: 204-574-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
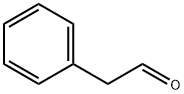
What is Phenylacetaldehyde?
Description
Phenylacetaldehyde is an organic compound which can be naturally found in buckwheat, flowers, and communication pheromones from various insect orders. It is commonly used for the preparation of fragrance as well as flavors, and applied in flavored cigarettes and beverages because of its honey-like sweet character. It is also applied in the synthesis of polymers, such as polyesters, as a rate controlling additive in polymerization reactions and used in the preparation of more complex chemicals like resmethrin, where it acts as a building block.
Chemical properties
Phenylacetaldehyde has a harsh, green odor reminiscent of hyacinth on dilution. It has an unpleasant, pungent, bitter flavor, turning sweet and fruit-like at low levels. Since it readily undergoes oxidation and polymerizes, it must be stabilized by addition of antioxidants and by dilution with, for example, diethyl phthalate before use in compositions.
Occurrence
Identified among the constituents of several essential oils: neroli, Citrus sinensis leaves, other citrus species (flowers and leaves), narcissus, magnolia, lily, rose and tea. It is reported found in over 170 natural products including apricot, sour cherry, cooked apple, peach, fresh blackberry, crispbread, other types of bread, green tea, unprocessed rice, lemon balm, red sage, black currant, bilberry, cranberry, other berries, grapes, raisins, melon, papaya, guava fruit, pineapple, asparagus, celery leaves, carrot, parsley, peas, bell pepper, sweet pepper, peach, cabbage, peppermint oil, Scotch spearmint oil, mustard, vinegar, onion, cooked potato, tomato, cinnamon bark, cassia leaf, ginger, many cheeses, milk, yogurt, boiled egg, cooked and cured meats, beer, cognac, grape wines, cocoa, coffee, tea, roasted filbert, roasted peanut, soybean, pecans, cauliflower, broccoli, honey, avocado, passion fruit, beans, mushrooms, trassi, mango, tamarind, rice, licorice, buckwheat, lovage root, pumpkin, sweet potato, cassava, corn oil, malt, wort, dried bonito, loquat, pawpaw, maté, sweet grass oil, orange peel oil, grapefruit juice, endive, clam and Chinese quince.
The Uses of Phenylacetaldehyde
Phenylacetaldehyde is used for the preparation of fragrances and polymers like polyesters, which find application as a rate controlling additive in polymerization reactions. It is an active component of fragrances and floral scent due to its honey-like sweet character and finds application in flavored cigarettes and beverages. It is an insect attractant and utilized in blacklight trap for pests. It is also used as a building block in the synthesis of more complex chemicals, such as resmethrin. Furthermore, it is used in association with acetic anhydride and allyltrimethylsilane in three-component coupling process catalyzed by LiBF4 providing homoallylic acetates.
What are the applications of Application
Phenylacetaldehyde is a naturally occurring aromatic compound
Definition
ChEBI: Phenylacetaldehyde is an aldehyde that consists of acetaldehyde bearing a methyl substituent; the parent member of the phenylacetaldehyde class of compounds. It has a role as a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is an alpha-CH2-containing aldehyde and a member of phenylacetaldehydes.
Aroma threshold values
Detection: 4 ppb. Aroma characteristics at 1.0%: sweet, floral honey, rosy and slightly powdery with a fermented note, cocoa and tobacco with a yellow tropical fruity nuance.
Taste threshold values
Taste characteristics at 5 ppm: floral and honey-like with a sweet floral, fruity, tobacco, with a yellow tropical fruity waxy nuance.
Synthesis Reference(s)
Journal of the American Chemical Society, 99, p. 4536, 1977 DOI: 10.1021/ja00455a071
Tetrahedron Letters, 29, p. 1471, 1988 DOI: 10.1016/S0040-4039(00)80328-7
General Description
Phenylacetaldehyde is an important aroma volatile found in tomato and roses. It has also been identified in potato, roasted cocoa beans and honey. Phenylacetaldehyde is also a potent moth attractant.
Biochem/physiol Actions
Phenylacetaldehyde is an insect attractant and can be used in blacklight trap for pests. It is constituent of floral scent. It is an intermediate in a variety of biochemical pathways.
Safety Profile
Moderately toxic by ingestion. Human skin irritant. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES.
Synthesis
Phenylacetaldehyde can be synthesized by Darzen glycidic ester synthesis from benzaldehyde; readily oxidizable to phenyl acetic acid.
Production Methods
The most important method industrially is the isomerization of styrene oxide. Ion-exchange resins, catalysis by chromium trioxide–tungsten trioxide on graphite or silicon dioxide–aluminum trioxide, and thermolysis are recommended for the rearrangement. Phenylacetaldehyde can also be synthesized by the direct oxidation of styrene in the presence of palladium salts and copper(II) chloride in aqueous solutions of glycol ethers. Other possibilities include the catalytic dehydrogenation of 2-phenylethanol on silver or gold catalysts, the hydroformylation of benzyl halides in the presence of dicobalt octacarbonyl and sodium carbonate in acetonitrile, and the Darzens glycide ester synthesis from benzaldehyde and alkyl chloroacetates.
References
https://en.wikipedia.org/wiki/Phenylacetaldehyde
https://www.alfa.com/zh-cn/catalog/A14263/
http://www.sigmaaldrich.com/catalog/product/aldrich/107395?lang=en®ion=US
Properties of Phenylacetaldehyde
| Melting point: | −10 °C(lit.) |
| Boiling point: | 195 °C |
| Density | 1.079 g/mL at 20 °C |
| vapor pressure | 2.09hPa at 20℃ |
| FEMA | 2874 | PHENYLACETALDEHYDE |
| refractive index | n |
| Flash point: | 188 °F |
| storage temp. | 2-8°C |
| solubility | 2.21g/l slightly soluble |
| form | Liquid |
| Specific Gravity | 1.075 (20/4℃) |
| color | Clear colorless to pale yellow |
| Odor | at 10.00 % in dipropylene glycol. green sweet floral hyacinth clover honey cocoa |
| Water Solubility | 2.210 g/L (25 ºC) |
| Sensitive | Air Sensitive |
| Merck | 14,7265 |
| JECFA Number | 1002 |
| BRN | 385791 |
| Dielectric constant | 4.8(20℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases. |
| CAS DataBase Reference | 122-78-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzeneacetaldehyde(122-78-1) |
| EPA Substance Registry System | Phenylacetaldehyde (122-78-1) |
Safety information for Phenylacetaldehyde
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H317:Sensitisation, Skin H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Phenylacetaldehyde
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 122-78-1 Phenyl Acetaldehyde 99%View Details
122-78-1 Phenyl Acetaldehyde 99%View Details
122-78-1 -
 122-78-1 98%View Details
122-78-1 98%View Details
122-78-1 -
 Phenyl Acetaldehyde 98%View Details
Phenyl Acetaldehyde 98%View Details
122-78-1 -
 Phenyl Acetaldehyde 122-78-1 98%View Details
Phenyl Acetaldehyde 122-78-1 98%View Details
122-78-1 -
 Phenylacetaldehyde (40-55% in Diethyl Phthalate) CAS 122-78-1View Details
Phenylacetaldehyde (40-55% in Diethyl Phthalate) CAS 122-78-1View Details
122-78-1 -
 Phenylacetaldehyde CAS 122-78-1View Details
Phenylacetaldehyde CAS 122-78-1View Details
122-78-1 -
 Phenylacetaldehyde 90 % CAS 122-78-1View Details
Phenylacetaldehyde 90 % CAS 122-78-1View Details
122-78-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
