Glyoxylic acid
Synonym(s):Formylformic acid;Oxoethanoic acid
- CAS NO.:298-12-4
- Empirical Formula: C2H2O3
- Molecular Weight: 74.04
- MDL number: MFCD00006958
- EINECS: 206-058-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-13 08:41:35
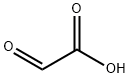
What is Glyoxylic acid?
Chemical properties
Colorless clear liquid.
The Uses of Glyoxylic acid
Glyoxalic acid can be used in the synthesis of a variety of reactions.
Glyoxylic acid is used in Hopkins Cole reaction, which is used in the detection of tryptophan in proteins.
It reacts with phenol to get 4-hydroxymandelic acid, which on further reaction with ammonia gives hydroxyphenylglycine, as a precursor to the drug amoxicillin.
It is also used as a starting material for the preparation of 4-hydroxyphenylacetic acid, which is used to get atenolol.
Definition
ChEBI: A 2-oxo monocarboxylic acid that is acetic acid bearing an oxo group at the alpha carbon atom.
General Description
Supplied as a 50% aqueous solution.
Health Hazard
Contact will cause severe eye and skin burns. Vapor exposure may cause eye and skin irritation.
Flammability and Explosibility
Non flammable
Biological Activity
nsc 27785 is an organic compound that is both an aldehyde and a carboxylic acid.
Synthesis
Add to 3500ml reactor equipped with reflux condenser, stirrer and thermometer300ml (3.55mol) of 50% glyoxal solution, stir and cool, control the temperature to 7±2,Slowly drip 300ml (3.55mol) of 35% hydrogen peroxide solution at the same time within 3 hours,280ml (0.355mol) of 17% ferrous sulfate solution and 120ml (0.75mol) of 15% aqueous ammonia solution, keep at 8±2, react for 3 hours, and let stand overnight, The reaction liquid was filtered to remove the solid by-products, and 350ml of water-carrying toluene was added to the reaction mother liquid.Heat to reflux, remove water, and distill toluene with water-carrying agent to obtain oxidation product.Add 120ml of concentrated sulfuric acid and 700ml of absolute ethanol to the oxidation products.Esterification reaction at 140°C for 3 hours, then distillation under reduced pressure, at 0.02Mpa,The esterification product was collected at 185°C.Add 120ml of 18% sulfuric acid to the esterified product and heat under stirring.The hydrolysis reaction is carried out at 95-98 for 2 hours; the hydrolysis liquid is cooled to room temperature,The precipitated white solid product was filtered, washed 4 times with 120ml of toluene each time, and dried.Obtain 180 g of the target product glyoxylic acid with a purity of 99.5% (HPLC) and a yield of 88%.Melting point is 98.1-98.6.
Source
Glyoxylic acid occurs in unripe fruit and in young green leaves. It has also been found in very young sugarbeets. Glyoxylic acid is found in plants and is a metabolite in mammalian biochemical pathways.
Environmental Fate
Glyoxylic acid's production and use as a cleaning agent for a variety of industrial applications, as a specialty chemical and biodegradable copolymer feedstock, and as an ingredient in cosmetics may result in its release to the environment through various waste streams. The pKa of glyoxylic acid is 3.3, indicating this compound will exist primarily as an anion in moist soil surfaces and anions are expected to have very high mobility in soils. If released to soil or water, glyoxylic acid is expected to biodegrade. Degradation may also occur in sunlit water through direct photolysis.
Properties of Glyoxylic acid
| Melting point: | -93°C |
| Boiling point: | 111°C |
| Density | 1.33 g/mL at 20 °C |
| vapor pressure | 14hPa at 19.85℃ |
| refractive index | n |
| Flash point: | 111°C |
| storage temp. | Store below +30°C. |
| solubility | Miscible with ethanol. Slightly miscible with ether and benzene. Immiscible with esters. |
| form | clear liquid |
| pka | 3.18(at 25℃) |
| color | Colorless to Light orange to Yellow |
| Water Solubility | miscible |
| Merck | 14,4511 |
| BRN | 741891 |
| CAS DataBase Reference | 298-12-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, oxo-(298-12-4) |
| EPA Substance Registry System | Acetic acid, 2-oxo- (298-12-4) |
Safety information for Glyoxylic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H290:Corrosive to Metals H317:Sensitisation, Skin H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Glyoxylic acid
| InChIKey | HHLFWLYXYJOTON-UHFFFAOYSA-N |
Glyoxylic acid manufacturer
JSK Chemicals
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 Glyoxylic acid 98%View Details
Glyoxylic acid 98%View Details
298-12-4 -
 298-12-4 Glyoxylic acid solution, 50% in water 99%View Details
298-12-4 Glyoxylic acid solution, 50% in water 99%View Details
298-12-4 -
 Glyoxylic acid solution CAS 298-12-4View Details
Glyoxylic acid solution CAS 298-12-4View Details
298-12-4 -
 Glyoxylic acid solution CAS 298-12-4View Details
Glyoxylic acid solution CAS 298-12-4View Details
298-12-4 -
 Glyoxylic acid, 50%, solution CAS 298-12-4View Details
Glyoxylic acid, 50%, solution CAS 298-12-4View Details
298-12-4 -
 Glyoxylic Acid (ca. 50% in Water, ca. 9mol/L) CAS 298-12-4View Details
Glyoxylic Acid (ca. 50% in Water, ca. 9mol/L) CAS 298-12-4View Details
298-12-4 -
 Glyoxylic acid CASView Details
Glyoxylic acid CASView Details -
 Glyoxylic acid solution CAS 298-12-4View Details
Glyoxylic acid solution CAS 298-12-4View Details
298-12-4
