Chloramphenicol hemisuccinate
- CAS NO.:3544-94-3
- Empirical Formula: C15H16Cl2N2O8
- Molecular Weight: 423.2
- MDL number: MFCD01683261
- EINECS: 2225900
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 20:41:25
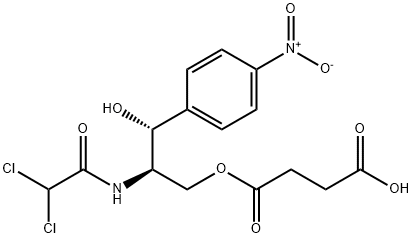
What is Chloramphenicol hemisuccinate?
Absorption
Chloramphenicol succinate has a high degree of interpatient variability, with a Tmax of 18 minutes to 3 hours. A 1g oral chloramphenicol succinate dose every 6-8 hours reaches a mean Cmax of 11.2μg/mL with a Tmax of 1 hour.
Toxicity
Patients experiencing an overdose may present with shock, cyanosis, and coma. Treat patients with symptomatic and supportive measures which may include administration of fluids, exchange transfusions, and administration of dopamine.
Description
Chloramphenicol succinate is a water-soluble prodrug form of the antibiotic chloramphenicol. It is a substrate for succinate dehydrogenase (SDH) and is oxidized by human liver and rat liver and kidney mitochondria to release chloramphenicol in vitro. Chloramphenicol succinate reduces human leukocyte migration in vitro. In vivo, chloramphenicol succinate reduces E. coli growth in rabbit and rat models of pyelonephritis when administered at doses of 150 and 200 mg/kg, respectively. Chloramphenicol succinate (20 mg/kg) reduces infarct size in a porcine model of myocardial ischemia-reperfusion injury. Formulations containing chloramphenicol succinate have been used in the treatment of severe bacterial infections.
The Uses of Chloramphenicol hemisuccinate
Chloramphenicol succinate is prepared by acylation of chloramphenicol with succinic anhydride to provide a water soluble pro-drug. Chloramphenicol succinate represents an alternative pro-drug strategy for chloramphenicol that has found a niche in surface antibiotic treatment in surgery. Chloramphenicol succinate is significantly less active than chloramphenicol but acts as a prodrug, forming chloramphenicol in the presence of succinate dehydrogenase.
Background
Chloramphenicol succinate is an ester prodrug of chloramphenicol. Chloramphenicol is a bacteriostatic antibiotic. Use of chloramphenicol succinate and chloramphenicol has decreased due to the risk of potentially fatal blood dyscrasias.
Chloramphenicol succinate was granted FDA approval on 20 February 1959.
What are the applications of Application
Chloramphenicol succinate is a prodrug of chloramphenicol
Indications
Chloramphenicol succinate is indicated to treat serious and susceptible bacterial infections where less dangerous drugs are ineffective or contraindicated.
Definition
ChEBI: Chloramphenicol succinate is a member of amphetamines and a hemisuccinate.
Pharmacokinetics
Chloramphenicol succinate is a prodrug of chloramphenicol, which binds to bacterial ribosomes and prevents translation. It has a narrow therapeutic index and a moderate duration of action. Patients should be counselled regarding the risk of serious fatal blood dyscrasias.
Metabolism
Chloramphenicol succinate is hydrolyzed to chloramphenicol. The propane-diol moiety of chloramphenicol can be metabolised through a number of pathways including glucuronidation, sulfate conjugation, phosphorylation, acetylation, and oxidation. The dichloroacetate moiety can be metabolised through hydrolysis of the amide group and dechlorination. The nitro functional group can also be metabolised to an aryl amine and aryl amide metabolite.
Properties of Chloramphenicol hemisuccinate
| Melting point: | 127 °C |
| Boiling point: | 716.3±60.0 °C(Predicted) |
| Density | 1.6769 (rough estimate) |
| refractive index | 1.7350 (estimate) |
| storage temp. | Store at -20°C |
| solubility | DMF: soluble; DMSO: soluble; Ethanol: soluble; Methanol: soluble |
| form | A solid |
| pka | 4.36±0.17(Predicted) |
Safety information for Chloramphenicol hemisuccinate
Computed Descriptors for Chloramphenicol hemisuccinate
Chloramphenicol hemisuccinate manufacturer
ShreeGen Pharma Ltd (part of Rumit Group of Companies)
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 3544-94-3 Chloramphenicol hemisuccinate 98%View Details
3544-94-3 Chloramphenicol hemisuccinate 98%View Details
3544-94-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8