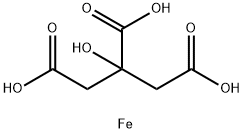
iron succinate
- CAS NO.:10030-90-7
- Empirical Formula: C4H4FeO4
- Molecular Weight: 171.91716
- MDL number: MFCD00672154
- EINECS: 233-082-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-17 20:33:23
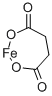
What is iron succinate?
Absorption
The efficiency of absorption depends on the salt form, the amount administered, the dosing regimen and the size of iron stores. Subjects with normal iron stores absorb 10% to 35% of an iron dose. Those who are iron deficient may absorb up to 95% of an iron dose.
Toxicity
Acute iron overdosage can be divided into four stages. In the first stage, which occurs up to six hours after ingestion, the principal symptoms are vomiting and diarrhea. Other symptoms include hypotension, tachycardia and CNS depression ranging from lethargy to coma. The second phase may occur at 6-24 hours after ingestion and is characterized by a temporary remission. In the third phase, gastrointestinal symptoms recur accompanied by shock, metabolic acidosis, coma, hepatic necrosis and jaundice, hypoglycemia, renal failure and pulmonary edema. The fourth phase may occur several weeks after ingestion and is characterized by gastrointestinal obstruction and liver damage. In a young child, 75 milligrams per kilogram is considered extremely dangerous. A dose of 30 milligrams per kilogram can lead to symptoms of toxicity. Estimates of a lethal dosage range from 180 milligrams per kilogram and upwards. A peak serum iron concentration of five micrograms or more per ml is associated with moderate to severe poisoning in many.
The Uses of iron succinate
Iron succinate is an oral iron supplement for the treatment of iron deficiency (ID). Iron succinate increases ferritin and transferrin saturation in non-anaemic heart failure and iron deficient patients and can significantly increase iron uptake in patients with heart failure (HF), nearly doubling ferritin levels and increasing TSAT. Patients with HFrEF or HFpEF appear to have a similar response[1].
Indications
Used in preventing and treating iron-deficiency anemia.
Pharmacokinetics
The major activity of supplemental iron is in the prevention and treatment of iron deficiency anemia. Iron has putative immune-enhancing, anticarcinogenic and cognition-enhancing activities.
Metabolism
Not Available
Storage
2-8°C
References
[1] K. BOMAN M. O. Iron Succinate Increased Ferritin and Transferrin Saturation in Non-Anaemic Patients with Heart Failure and Iron Deficiency—A Pilot Study[J]. World Journal of Cardiovascular Diseases, 2021, 11 1: 11-19. DOI:10.4236/WJCD.2021.111002.
Safety information for iron succinate
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

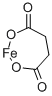



You may like
-
 10030-90-7 Ferrous succinate 99%View Details
10030-90-7 Ferrous succinate 99%View Details
10030-90-7 -
 10030-90-7 98%View Details
10030-90-7 98%View Details
10030-90-7 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
