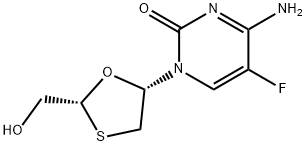
CEVIMELINE, HYDROCHLORIDE SALT
- CAS NO.:107233-08-9
- Empirical Formula: C10H17NOS
- Molecular Weight: 199.31
- MDL number: MFCD01961045
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 09:32:30

What is CEVIMELINE, HYDROCHLORIDE SALT?
Absorption
Rapidly absorbed with peak concentration after 1.5 to 2 hours
Chemical properties
Off-White Solid
The Uses of CEVIMELINE, HYDROCHLORIDE SALT
A muscarinic M1 and M3 receptor agonist. Sialagogue
Background
Cevimeline is a parasympathomimetic agent that act as an agonist at the muscarinic acetylcholine receptors M1 and M3. It is indicated by the Food and Drug Administration for the treatment of dry mouth associated with Sj?gren's syndrome.
Indications
For the treatment of symptoms of dry mouth in patients with Sj?gren's Syndrome.
Biological Activity
cevimeline is a muscarinic receptor agonist especially on the m1 and m3 receptors. [1]cevimeline has been approved for use against symptoms of dry mouth by activating the m3 receptors of the parasympathetic nervous system. cevimeline is effective and safe in improving symptoms of dry eye with 20 mg three times per day [2]. cevimeline increased the intracellular ca+ level in parotid gland acinar cells over 1 μm and rat, enhanced the excitability via muscarinic receptors, thereby, cevimeline alleviates dry mouth symptoms by stimulating secretion by the salivary glands. cevimeline has a longer duration of salivation[3]. cevimeline plays a part in alzheimer’s disease. cevimeline decreased aβ (1–40) level in the cerebrospinal fluid (csf) at 1 mg/kg without changing α-apps in rabbit and significantly decreased csf aβ in ad patients.[4]
Pharmacokinetics
Cevimeline is a cholinergic agonist which binds to muscarinic receptors. Muscarinic agonists in sufficient dosage can increase secretion of exocrine glands, such as salivary and sweat glands and increase tone of the smooth muscle in the gastrointestinal and urinary tracts.
Metabolism
Primarily hepatic, isozymes CYP2D6 and CYP3A4 are responsible for the metabolism of cevimeline. Approximately 44.5% of the drug is converted to cis and trans-sulfoxide, 22.3% to glucuronic acid conjugate, and 4% to N-oxide of cevimeline. Approximately 8% of the trans-sulfoxide metabolite is then converted into the corresponding glucuronic acid conjugate.
References
1. f. b. vivino, i. al-hashimi, z. khan, f. g. leveque, p. l. salisbury, 3rd, t. k. tran-johnson, c. c. muscoplat, m. trivedi, b. goldlust and s. c. gallagher, arch intern med 1999, 159, 174-181. 2. m. ono, e. takamura, k. shinozaki, t. tsumura, t. hamano, y. yagi and k. tsubota, am j ophthalmol 2004, 138, 6-17. 3. k. ono, t. inagaki, t. iida, r. hosokawa and k. inenaga, j med invest 2009, 56 suppl, 375. 4. a. fisher, z. pittel, r. haring, n. bar-ner, m. kliger-spatz, n. natan, i. egozi, h. sonego, i. marcovitch and r. brandeis, j mol neurosci 2003, 20, 349-356.
Properties of CEVIMELINE, HYDROCHLORIDE SALT
| Melting point: | 195-197°C |
| Boiling point: | 308.5±42.0 °C(Predicted) |
| Density | 1.19 |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | 9.51±0.40(Predicted) |
| CAS DataBase Reference | 107233-08-9 |
Safety information for CEVIMELINE, HYDROCHLORIDE SALT
Computed Descriptors for CEVIMELINE, HYDROCHLORIDE SALT
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 107233-08-9 Cevimeline 98%View Details
107233-08-9 Cevimeline 98%View Details
107233-08-9 -
 Cevimeline 107233-08-9 98%View Details
Cevimeline 107233-08-9 98%View Details
107233-08-9 -
 Cevimeline 107233-08-9 98%View Details
Cevimeline 107233-08-9 98%View Details
107233-08-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6