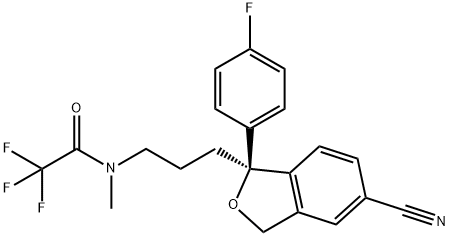
Citalopram
- CAS NO.:59729-33-8
- Empirical Formula: C20H21FN2O
- Molecular Weight: 324.39
- MDL number: MFCD00865398
- EINECS: 261-891-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
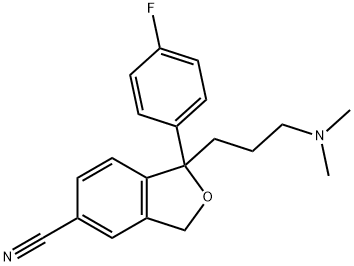
What is Citalopram?
Absorption
The single- and multiple-dose pharmacokinetics of citalopram are linear and dose-proportional in a dose range of 10 to 40 mg/day. Biotransformation of citalopram is mainly hepatic, with a mean terminal half-life of about 35 hours. With once daily dosing, steady state plasma concentrations are achieved within approximately one week. At steady state, the extent of accumulation of citalopram in plasma, based on the half-life, is expected to be 2.5 times the plasma concentrations observed after a single dose.
Following a single oral dose (40 mg tablet) of citalopram, peak blood levels occur at about 4 hours. The absolute bioavailability of citalopram was about 80% relative to an intravenous dose, and absorption is not affected by food.
Toxicity
Based on data from published observational studies, exposure to SSRIs, particularly in the month before delivery, has been associated with a less than 2-fold increase in the risk of postpartum hemorrhage.
Available data from published epidemiologic studies and postmarketing reports with citalopram use in pregnancy have not established an increased risk of major birth defects or miscarriage. Published studies demonstrated that citalopram levels in both cord blood and amniotic fluid are similar to those observed in maternal serum. There are risks of persistent pulmonary hypertension of the newborn (PPHN) and/or poor neonatal adaptation with exposure to selective serotonin reuptake inhibitors (SSRIs), including citalopram, during pregnancy. There also are risks associated with untreated depression in pregnancy.
Citalopram was administered orally to pregnant rats during the period of organogenesis at doses of 32, 56, and 112 mg/kg/day, which are approximately 8, 14, and 27 times the Maximum Recommended Human Dose (MRHD) of 40 mg, based on mg/m2 body surface area. Citalopram caused maternal toxicity of CNS clinical signs and decreased weight gain at 112 mg/kg/day, which is 27 times the MRHD. At this maternally toxic dose, citalopram decreased embryo/fetal growth and survival and increased fetal abnormalities (including cardiovascular and skeletal defects). The no observed adverse effect level (NOAEL) for maternal and embryofetal toxicity is 56 mg/kg/day, which is approximately 14 times the MRHD.
Citalopram was administered orally to pregnant rabbits during the period of organogenesis at doses up to 16 mg/kg/day, which is approximately 8 times the MRHD of 40 mg, based on mg/m2 body surface area. No maternal or embryofetal toxicity was observed. The NOAEL for maternal and embryofetal toxicity is 16 mg/kg/day, which is approximately 8 times the MRHD.
Citalopram was administered orally to pregnant rats during late gestation and lactation periods at doses of 4.8, 12.8, and 32 mg/kg/day, which are approximately 1, 3, and 8 times the MRHD of 40 mg, based on mg/m2 body surface area. Citalopram increased offspring mortality during the first 4 days of birth and decreased offspring growth at 32 mg/kg/day, which is approximately 8 times the MRHD. The NOAEL for developmental toxicity is 12.8 mg/kg/day, which is approximately 3 times the MRHD. In a separate study, similar effects on offspring mortality and growth were seen when dams were treated throughout gestation and early lactation at doses ≥ 24 mg/kg/day, which is approximately 6 times the MRHD. A NOAEL was not determined in that study.
SSRIs, including citalopram, have been associated with cases of clinically significant hyponatremia in elderly patients,
who may be at greater risk for this adverse reaction.
The following have been reported with citalopram tablet overdosage:
? Seizures, which may be delayed, and altered mental status including coma.
? Cardiovascular toxicity, which may be delayed, including QRS and QTc interval prolongation, wide complex tachyarrhythmias, and torsade de pointes. Hypertension is most commonly seen, but hypotension can rarely be seen alone or with co‐ingestants including alcohol.
? Serotonin syndrome (patients with a multiple drug overdosage with other pro-serotonergic drugs may have a higher risk).
Prolonged cardiac monitoring is recommended in citalopram overdosage ingestions due to the arrhythmia risk. Gastrointestinal decontamination with activated charcoal should be considered in patients who present early after a citalopram overdose. Consider contacting a Poison Center (1‐800‐221‐2222) or a medical toxicologist for additional overdosage management recommendations.
Citalopram increased the incidence of small intestine carcinoma in rats treated for 24 months at doses of 8 and 24 mg/kg/day in the diet, which are approximately 2 and 6 times the Maximum Recommended Human Dose (MRHD) of 40 mg, respectively, based on mg/m2 body surface area. A no-effect level (NOEL) for this finding was not established. Citalopram did not increase the incidence of tumors in mice treated for 18 months at doses up to 240 mg/kg/day in the diet, which is approximately 30 times the MRDH of 40 mg based on mg/m2 body surface area.
Citalopram was mutagenic in the in vitro bacterial reverse mutation assay (Ames test) in 2 of 5 bacterial strains (Salmonella TA98 and TA1537) in the absence of metabolic activation. It was clastogenic in the in vitro Chinese hamster lung cell assay for chromosomal aberrations in the presence and absence of metabolic activation. Citalopram was not mutagenic in the in vitro mammalian forward gene mutation assay (HPRT) in mouse lymphoma cells or in in vitro/in vivo unscheduled DNA synthesis (UDS) assay in rat liver. It was not clastogenic in the in vitro chromosomal aberration assay in human lymphocytes or in two in vivo mouse micronucleus assays.
Citalopram was administered orally to female and male rats at doses of 32, 48, and 72 mg/kg/day prior to and throughout mating and continuing to gestation. These doses are approximately 8, 12, and 17 times the MRHD of 40 mg based on mg/m2 body surface area. Mating and fertility were decreased at doses ≥ 32 mg/kg/day, which is approximately 8 times the MRHD. Gestation duration was increased to 48 mg/kg/day, which is approximately 12 times the MRHD.
Description
Citalopram is a specific serotonin-uptake inhibitor useful in the treatment of depression. In endogenous depression citalopram was reported to be as effective as amitriptyline and mianserin, while being inferior to clomipramine in both endogenous and non-endogenous depression.
Originator
Lundbeck (Denmark)
The Uses of Citalopram
scabicide
The Uses of Citalopram
antidepressant monoamine oxidase inhibitor (MAOIs)
Background
Citalopram is an antidepressant belonging to the class of selective serotonin-reuptake inhibitors (SSRIs) widely used to treat the symptoms of depression. It is a racemic bicyclic phthalate derivate and is the only compound with a tertiary amine and 2 nitrogen-containing metabolites among all SSRIs. Citalopram enhances serotonergic transmission through the inhibition of serotonin reuptake, and among all the SSRIs, citalopram appears to be the most selective toward serotonin reuptake inhibition. Specifically, it has a very minimal effect on dopamine and norepinephrine transportation and virtually no affinity for muscarinic, histaminergic, or GABAergic receptors.
Citalopram was approved by the FDA in 1998 for the treatment of depression in adults 18 years or older.
Indications
Citalopram is approved by the FDA for treating adults with major depressive disorder. It has also been used off-label to treat various diseases, including but not limited to sexual dysfunction, ethanol abuse, psychiatric conditions such as obsessive-compulsive disorder (OCD), social anxiety disorder, panic disorder, and diabetic neuropathy.
Definition
ChEBI: A nitrile that is 1,3-dihydro-2-benzofuran-5-carbonitrile in which one of the hydrogens at position 1 is replaced by a p-fluorophenyl group, while the other is replaced by a 3-(dimethylamino)propyl group.
brand name
Cipramil
Biological Functions
Citalopram (Celexa) has an elimination half-life of 35 hours and is 80% bound to plasma proteins. Of all of the SSRIs it has the least effect on the cytochrome P450 system and has the most favorable profile regarding drug–drug interactions.
General Description
Citalopram (Celexa) is a racemic mixture and is very SERTselective. The N-monodemethylated compound is slightlyless potent but is as selective. The aryl substituents are importantfor activity. The ether function is important andprobably interacts with the protonated amino group to givea suitable shape for SERT binding.
Biological Activity
Highly selective and potent 5-HT uptake inhibitor with no effect on noradrenalin or dopamine uptake (IC 50 values are 1.8, 8800 and 41000 nM respectively). Has negligible activity at a wide range of receptors and is clinically used as an antidepressant. Also available as part of the Serotonin Uptake Inhibitor Tocriset™ .
Pharmacokinetics
Citalopram belongs to a class of antidepressants known as selective serotonin reuptake inhibitors (SSRIs). It has been found to relieve or manage symptoms of depression, anxiety, eating disorders and obsessive-compulsive disorder among other mood disorders. The antidepressant, anti-anxiety, and other actions of citalopram are linked to its inhibition of CNS central uptake of serotonin. Serotonergic abnormalities have been reported in patients with mood disorders. Behavioral and neuropsychological effects of serotonin include the regulation of mood, perception, reward, anger, aggression, appetite, memory, sexuality, and attention, as examples. The onset of action for depression is approximately 1 to 4 weeks. The complete response may take 8-12 weeks after initiation of citalopram.
In vitro studies demonstrate that citalopram is a strong and selective inhibitor of neuronal serotonin reuptake and has weak effects on norepinephrine and dopamine central reuptake. The chronic administration of citalopram has been shown to downregulate central norepinephrine receptors, similar to other drugs effective in the treatment of major depressive disorder. Citalopram does not inhibit monoamine oxidase.
Clinical Use
SSRI antidepressant:
Depressive illness
Panic disorder
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: increased risk of bleeding with aspirin
and NSAIDs; risk of CNS toxicity increased with
tramadol.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with amiodarone, disopyramide and
dronedarone - avoid.
Antibacterials: possibly increased risk of ventricular
arrhythmias with IV erythromycin, moxifloxacin,
pentamidine and telithromycin.
Anticoagulants: effect of coumarins possibly
enhanced; possibly increased risk of bleeding with
dabigatran.
Antidepressants: avoid with MAOIs and
moclobemide, increased risk of toxicity; avoid with
St John’s wort; possibly enhanced serotonergic
effects with dapoxetine and duloxetine; can increase
tricyclics antidepressant concentration; increased
agitation and nausea with tryptophan; possible
increased risk of convulsions with vortioxetine.
Antiepileptics: convulsive threshold lowered.
Antihistamines: increased risk of ventricular
arrhythmias with mizolastine - avoid.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol; possible increased
risk of ventricular arrhythmias with chloroquine and
quinine.
Antipsychotics: possibly increased clozapine
concentration; increased risk of ventricular
arrhythmias with haloperidol and pimozide - avoid.
Antivirals: concentration possibly increased by
ritonavir.
Beta-blockers: increased risk of ventricular
arrhythmias with sotalol - avoid.
Dopaminergics: avoid with selegiline; increased risk
of CNS toxicity with rasagiline.
5 HT1
agonist: increased risk of CNS toxicity -
avoid; possibly increased risk of serotonergic effects
with naratriptan.
Linezolid: use with care, possibly increased risk of
side effects.
Lithium: increased risk of CNS effects.
Methylthioninium: risk of CNS toxicity - avoid if
possible.
Metabolism
Citalopram is metabolized mainly in the liver via N-demethylation to its main metabolite, demethylcitalopram by CYP2C19 and CYP3A4. Other metabolites include didemethylcitalopram via CYP2D6 metabolism, citalopram N-oxide and propionic acid derivative via monoamine oxidase enzymes A and B and aldehyde oxidase. Citalopram metabolites exert little pharmacologic activity in comparison to the parent drug and are not likely to contribute to the clinical effect of citalopram.
Metabolism
Citalopram is metabolised by demethylation, deamination, and oxidation to active and inactive metabolites. The demethylation of citalopram to one of its active metabolites, demethylcitalopram, involves the cytochrome P450 isoenzymes CYP3A4 and CYP2C19; the metabolism of citalopram is also partly dependent on CYP2D6. Didemethylcitalopram has also been identified as a metabolite of citalopram. It is excreted mainly via the liver (85%) with the remainder via the kidneys. About 12% is excreted in the urine as unchanged drug.
Properties of Citalopram
| Melting point: | 156-157 °C |
| Boiling point: | bp0.03 175-181° |
| Density | 1.18±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | Solid |
| pka | pKa 9.38(H2O) (Uncertain) |
| color | White to Off-White |
| CAS DataBase Reference | 59729-33-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Citalopram(59729-33-8) |
Safety information for Citalopram
Computed Descriptors for Citalopram
Citalopram manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 59729-33-8 98%View Details
59729-33-8 98%View Details
59729-33-8 -
 Citalopram 98%View Details
Citalopram 98%View Details -
 Citalopram 59729-33-8 98%View Details
Citalopram 59729-33-8 98%View Details
59729-33-8 -
 59729-33-8 98%View Details
59729-33-8 98%View Details
59729-33-8 -
 Citalopram 98%View Details
Citalopram 98%View Details
59729-33-8 -
 Citalopram 59729-33-8 98%View Details
Citalopram 59729-33-8 98%View Details
59729-33-8 -
 Citalopram 98%View Details
Citalopram 98%View Details
59729-33-8 -
 Citalopram Hydrobrom 99%View Details
Citalopram Hydrobrom 99%View Details
