Formoterol
- CAS NO.:73573-87-2
- Empirical Formula: C19H24N2O4
- Molecular Weight: 344.4
- MDL number: MFCD05662345
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-19 15:37:50
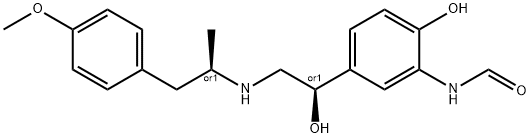
What is Formoterol?
Absorption
The pulmonary bioavailability of formoterol has been estimated to be about 43% of the delivered dose, while the total systemic bioavailability is approximately 60% of the delivered dose (as systemic bioavailability accounts for absorption in the gut).
Formoterol is rapidly absorbed into plasma following inhalation. In healthy adults, formoterol Tmax ranged from 0.167 to 0.5 hours. Following a single dose of 10 mcg, Cmax and AUC were 22 pmol/L and 81 pmol.h/L, respectively. In asthmatic adult patients, Tmax ranged from 0.58 to 1.97 hours. Following single-dose administration of 10mcg, Cmax and AUC0-12h were 22 pmol/L and 125 pmol.h/L, respectively; following multiple-dose administration of 10 mcg, Cmax and AUC0-12h were 41 pmol/L and 226 pmol.h/L, respectively. Absorption appears to be proportional to dose across standard dosing ranges.
Toxicity
The oral LD50 in rats is 3130 mg/kg.
Symptoms of overdose are likely consistent with formoterol's adverse effect profile (i.e. consistent with excessive beta-adrenergic stimulation) and may include angina, hyper or hypotension, tachycardia, arrhythmia, nervousness, headache, tremor, seizures, dry mouth, etc. Patients may experience laboratory abnormalities including hypokalemia, hyperglycemia, and metabolic acidosis. Treatment of overdosage should consist of symptomatic and supportive therapy, with a particular focus on cardiac monitoring. Consider the use of a cardioselective beta-adrenergic blocker to oppose excessive adrenergic stimulation if clinically appropriate.
Description
Formoterol is a potent bronchoselective β-agonist with long duration of action given by inhalation.
Chemical properties
solid
The Uses of Formoterol
Labeled Formoterol, intended for use as an internal standard for the quantification of Formoterol by GC- or LC-mass spectrometry.
The Uses of Formoterol
Formoterol is a useful compound for treating respiratory obstructive diseases.
Indications
Formoterol is indicated in various formulations for the treatment of asthma and COPD. For the treatment of COPD, formoterol is available as a single-entity inhalation solution, in combination with the long-acting muscarinic antagonists (LAMAs) aclidinium and glycopyrronium, and in combination with the corticosteroid budesonide. For the treatment of asthma, formoterol is available in combination with mometasone furoate for patients 5 years and older and with budesonide for patients 6 years and older. Formoterol may also be used on an as-needed basis for prophylaxis against exercise-induced bronchospasm.
Background
Formoterol is an inhaled beta2-agonist used in the management of COPD and asthma that was first approved for use in the United States in 2001. It acts on bronchial smooth muscle to dilate and relax airways, and is administered as a racemic mixture of its active (R;R)- and inactive (S;S)-enantiomers. A major clinical advantage of formoterol over other inhaled beta-agonists is its rapid onset of action (2-3 minutes), which is at least as fast as salbutamol, combined with a long duration of action (12 hours) - for this reason, treatment guidelines for asthma recommend its use as both a reliever and maintenance medication. It is available as a single-entity product and in several formulations in combination with both inhaled corticosteroids and long-acting muscarinic antagonists.
Definition
ChEBI: A phenylethanoloamine having 4-hydroxy and 3-formamido substituents on the phenyl ring and an N-(4-methoxyphenyl)propan-2-yl substituent.
brand name
Foradil (Novartis).
General Description
Formoterol (Foradil) isalso a lipophilic (log P= 1.6) and long-acting β2-agonist. Ithas 3'-formylamino ( β-directing) and 4 OH groups on onephenyl ring and a lipophilic -directing N-isopropyl-pmethoxyphenylgroup on the nitrogen atom. Its long DOA(12 hours), which is comparable to that of salmeterol, hasbeen suggested to result from its association with the membranelipid bilayer, from which it gradually diffuses to provideprolonged stimulation of β2 receptors and its resistanceto MAO and COMT. Formoterol has a much faster onset ofaction than does salmeterol as result of its lower lipophilicity.Both of these long-acting drugs are used by inhalationand are recommended for maintenance treatment of asthma,usually in conjunction with an inhaled corticosteroid.
Mechanism of action
Formoterol has 3′-formylamino and 4′-hydroxy ring R3 substitution pattern but also an alkoxyphenylethyl lipophilic group R1 on the nitrogen, similar to ritodrine. Although it is less lipophilic (log P = 1.6) than salmeterol, it has a 12-hour duration of action similar to that for salmeterol. It is administered as an inhaled dry powder, because it is unstable to heat and moisture. It is a mixture of (R,R)-(–)- and (S,S)-(+)-stereoisomers, with the (R,R)-isomer having approximately 1,000-fold more affinity for the β2-receptor than the (S,S)-isomer. Because of its high potency and low dose, however, there is no clinical advantage for using (R,R)-formoterol as bronchodilator compared to the racemate. Unlike salmeterol, formoterol has a faster onset of action as a result of its lower lipophilicity. It is also more potent; a 12-μg dose of formoterol has been demonstrated to be equivalent to a 50-μg dose of salmeterol.
Pharmacokinetics
Formoterol works locally in the lungs as a bronchodilator, relaxing smooth muscle and opening up the airways. It possesses both a rapid onset of action (approximately 2-3 minutes) and a long duration of action (up to 12 hours). The use of long-acting beta-agonists (LABAs), such as formoterol, without concomitant inhaled corticosteroids in asthmatic patients should be avoided, as LABA monotherapy has been associated with an increased risk of asthma-related death.
Metabolism
Formoterol is metabolized primarily via direct glucuronidation of the parent drug and via O-demethylation of the parent drug followed by glucuronidation. Minor pathways include sulfate conjugation of the parent drug and deformylation of the parent drug followed by sulfate conjugation, though these minor pathways have not been fully characterized. The major pathway of formoterol metabolism is a direct glucuronidation of the parent drug at its phenolic hydroxyl group, while the second most prominent pathway involves O-demethylation following by glucuronidation at the phenolic hydroxyl group.
In vitro studies of formoterol disposition indicate that O-demethylation of formoterol involves a number of cytochrome P450 isoenzymes (CYP2D6, CYP2C19, CYP2C9, and CYP2A6) and glucuronidation involves a number of UDP-glucuronosyltransferase isoenzymes (UGT1A1, UGT1A8, UGT1A9, UGT2B7, and UGT2B15), though specific roles for individual enzymes have not been elucidated.
Properties of Formoterol
| Boiling point: | 603.2±55.0 °C(Predicted) |
| Density | 1.233±0.06 g/cm3(Predicted) |
| solubility | DMSO: 20 mg/mL, soluble |
| form | solid |
| pka | 8.95±0.50(Predicted) |
| color | off-white |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 73573-87-2(CAS DataBase Reference) |
Safety information for Formoterol
Computed Descriptors for Formoterol
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






You may like
-
 Formoterol 98% (HPLC) CAS 73573-87-2View Details
Formoterol 98% (HPLC) CAS 73573-87-2View Details
73573-87-2 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
