Levocetirizine
- CAS NO.:130018-77-8
- Empirical Formula: C21H25ClN2O3
- Molecular Weight: 388.89
- MDL number: MFCD04112703
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
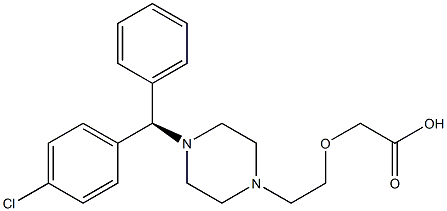
What is Levocetirizine?
Absorption
Following a 5mg oral dose of levocetirizine, a Cmax of 0.27±0.04μg/mL with a Tmax of 0.75±0.50h. The AUC of levocetirizine is 2.31±0.50μg*h/mL. Taking levocetirizine with food does not affect the AUC but delays Tmax by 1.25 hours and lowers Cmax by 36%.
Toxicity
Patients experiencing an overdose may present with drowsiness. Children may become agitated and restless before drowsiness. Patients should be treated with supportive measures. Dialysis will not assist in removing the drug from the body.
The maximal nonlethal dose in mice and rats is 240mg/kg.
Description
The (R)-enantiomer of the second-generation antihistamine cetirizine, levocetirizine, was first introduced in Germany for seasonal allergic rhinitis (including ocular symptoms), perennial allergic rhinitis and chronic idiopathic urticaria. The dihydrochloride salt can be prepared in four steps from optically active 4-chlorobenzhydrylamine obtained by resolution of its racemate with (+)-tartaric acid. Levocetirizine (eutomer) is a 2-fold more potent H1 antagonist than cetirizine whereas the other enantiomer (distomer) is 10-fold less potent compared to levocetirizine. Pharmacodynamic studies on healthy volunteers showed that compared to cetirizine, half the dose of levocetirizine (5 mg) was necessary to obtain similar inhibitory effects in the skin test of histamine-induced wheal and flare as well as on histamine-induced nasal congestion and nasal resistance. There was no evidence of chiral inversion of levocetirizine in vivo in several species including human. The daily dose of drug is rapidly and extensively absorbed in human. Interestingly, its volume of distribution (0.41 kg/L) is smaller than that of the distomer (0.60 kg/L). The low volume of distribution is considered as favorable for an antihistamine both in terms of efficacy and safety. Due to its high metabolic stability and lack of effect on the activities of the major CYP isoenzymes, levocetirizine is unlikely to cause interactions with other administered drugs. No clinically relevant effect on electrocardiograms of healthy volunteers was detected.
Chemical properties
Off-White Solid
Originator
Sepracor (US)
The Uses of Levocetirizine
A nonsedating type histamine H1-receptor antagonist. A major metabolite of Hydroxyzine. Pharmacological activity resides primarily in the (R)-isomer. Antihystaminic.
The Uses of Levocetirizine
H1 antihistamine, antiallergic
The Uses of Levocetirizine
Labeled cetirizine, intended for use as an internal standard for the quantification of cetirizine by GC- or LC-mass spectrometry.
What are the applications of Application
(R)-Cetirizine Dihydrochloride is a histamine H1-receptor antagonist and a metabolite of Hydroxyzine
Background
Levocetirizine is a selective histamine H1 antagonist used to treat a variety of allergic symptoms. It is the R enantiomer of cetirizine. Levocetirizine has greater affinity for the histamine H1 receptor than cetirizine.
Levocetirizine was granted FDA approval in 1995.
Indications
Levocetirizine is indicated to treat symptoms of perennial allergic rhinitis and uncomplicated skin manifestations of chronic idiopathic urticaria. It is also used over the counter for a variety of mild allergy symptoms.
Definition
ChEBI: 2-[2-[4-[(R)-(4-chlorophenyl)-phenylmethyl]-1-piperazinyl]ethoxy]acetic acid is a diarylmethane.
brand name
Xusal
Pharmacokinetics
Levocetirizine is a second generation histamine H1 antagonist used to treat various allergic symptoms. It has a long duration of action as it is generally taken once daily, and a wide therapeutic window as animal studies show the maximal nonlethal dose is over 100x a normal dose. Patients are cautioned to avoid tasks that require complete alertness, avoid alertness, and use caution in patients with factors predisposing urinary retention.
Clinical Use
Antihistamine:
Symptomatic relief of allergy such as hay fever,
urticaria
Metabolism
Levocetirizine is poorly metabolized with 85.8% of an oral dose being excreted as the unchanged drug. Levocetirizine can be metabolized to a dihydrodiol (M2), an N-oxide (M3), a hydroxymethoxy derivative (M4), a hydroxy derivative (M5), an O-dealkylated derivative (M6), a taurine conjugate (M8), and an N-dealkylated and aromatic hydroxylated derivative (M9). The M5 metabolite can be glucuronidated to form the M1 metabolite and the M9 metabolite can form 4-chloro-4'-hydroxybenzhydryl mercapturates (M10a and M10b).
Properties of Levocetirizine
| Melting point: | 205-208°C (dec.) |
| Boiling point: | 542.1±45.0 °C(Predicted) |
| Density | 1.237±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO: 78 mg/mL (200.57 mM);Ethanol: 78 mg/mL (200.57 mM) |
| pka | 3.46±0.10(Predicted) |
| form | Solid |
| color | White to off-white |
| Water Solubility | Water: 78 mg/mL (200.57 mM) |
| CAS DataBase Reference | 130018-77-8(CAS DataBase Reference) |
Safety information for Levocetirizine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Levocetirizine
Levocetirizine manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran




You may like
-
 130018-77-8 Levocetirizine-IP/IHS 98%View Details
130018-77-8 Levocetirizine-IP/IHS 98%View Details
130018-77-8 -
 Levocetirizine Hydrochloride IP / USP Complies as per MonographView Details
Levocetirizine Hydrochloride IP / USP Complies as per MonographView Details
130018-77-8 -
 130018-77-8 Levocetrizine 98%View Details
130018-77-8 Levocetrizine 98%View Details
130018-77-8 -
 130018-77-8 Levocetirizine 98%View Details
130018-77-8 Levocetirizine 98%View Details
130018-77-8 -
 130018-77-8 99%View Details
130018-77-8 99%View Details
130018-77-8 -
 Levocetirizine 130018-77-8 98%View Details
Levocetirizine 130018-77-8 98%View Details
130018-77-8 -
 130018-77-8 98%View Details
130018-77-8 98%View Details
130018-77-8 -
 Levocetirizine 130018-77-8 98%View Details
Levocetirizine 130018-77-8 98%View Details
130018-77-8
