Ceftizoxime sodium
Synonym(s):Ceftizoxime sodium
- CAS NO.:68401-82-1
- Empirical Formula: C13H14N5NaO5S2
- Molecular Weight: 407.39
- MDL number: MFCD01682036
- EINECS: 629-729-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 21:30:20
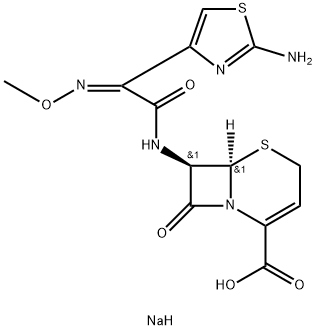
What is Ceftizoxime sodium?
Description
Ceftizoxime was synthesized by Fujisawa Pharmaceutical Industries in 1979. It possesses the (iminothiazolyl)methoxyiminomethyl group at the 7 position of the cephem nucleus, but there is no side chain at the 3 position. The compound shows excellent activity against gram-positive and gram-negative bacteria, behavior similar to that of cefotaxime. Unlike cefotaxime, however, it is not metabolized in vivo.
Chemical properties
Cefotaxime Sodium is white, white or light yellow crystalline powder; odorless; insoluble in organic solvents but soluble in water. The 10% solution has a pH of 4.5 to 6.5. Dilute solution is colorless or yellowish while high-concentration solution is grayish yellow. Dark yellow or brown color indicates that the drug has been deteriorated. The aqueous solution can be stored at 5 ℃ for 1 week.
Originator
Eposelin,Fujisawa,Japan,1982
The Uses of Ceftizoxime sodium
Antibacterial.
The Uses of Ceftizoxime sodium
A broad spectrum antibiotic targeting a wide variety of Gram-positive and Gram-negative bacteria
Definition
ChEBI: The sodium salt of ceftizoxime.
Manufacturing Process
Phosphorus oxychloride (2.0 g) was added at one time at 5°C to 10°C to a
suspension of 2-methoxyimino-2-(2-amino-1,3-thiazol-4-yl)acetic acid (syn
isomer) (2 g) in dry ethyl acetate (20 ml). After stirring for 20 minutes at 7°C
to 10°C, bis(trimethylsilyl)acetamide (0.4 g) was added thereto at the same
temperature. After stirring for 10 minutes at 7°C to 10°C, phosphorus
oxychloride (2.0 g) was dropwise added thereto at the same temperature. The
resulting mixture was stirred for 10 minutes at 7°C to 10°C, and dry
dimethylformamide (0.8 g) was dropwise added thereto at the same
temperature. The mixture was stirred for 30 minutes at 7°C to 10°C to give a
clear solution. On the other hand, trimethylsilylacetamide (7.35 g) was added
to a suspension of 7-aminocephalosporanic acid (2.45 g) in dry ethyl acetate
(8 ml), after which the mixture was stirred at 40°C to give a clear solution.
To this solution was added at one time the above-obtained ethyl acetate
solution at -15°C, and the resulting mixture was stirred for 1 hour at -10°C to
-15°C. The reaction mixture was cooled to -30°C, and water (80 ml) was
added thereto. The aqueous layer was separated, adjusted to pH 4.5 with
sodium bicarbonate and subjected to column chromatography on Diaion HP-20
resin (Mitsubishi Chemical Industries Ltd.) using 25% aqueous solution of
isopropyl alcohol as an eluent. The eluate was lyophilized to give 7-[2-
methoxyimino-2-(2-amino-1,3-thiazol-4-yl)acetamido]cephalosporanic acid
(syn isomer) (1.8 g), MP 227°C (decomp.).
brand name
Cefizox (Astellas).
Therapeutic Function
Antibacterial
Clinical Use
Ceftizoxime (Cefizox) is a third-generation cephalosporinthat was introduced in 1984. This β-lactamase–resistant agentexhibits excellent activity against the Enterobacteriaceae,especially E. coli, K. pneumoniae, E. cloacae, Enterobacteraerogenes, indole-positive and indole-negative Proteus spp.,and S. marcescens. Ceftizoxime is claimed to be more activethan cefoxitin against B. fragilis. It is also very active againstGram-positive bacteria. Its activity against P. aeruginosa issomewhat variable and lower than that of either cefotaxime orcefoperazone.
Ceftizoxime is not metabolized in vivo. It is excretedlargely unchanged in the urine. Adequate levels of the drugare achieved in the cerebrospinal fluid for the treatment ofGram-negative or Gram-positive bacterial meningitis. It mustbe administered on a thrice-daily dosing schedule because ofits relatively short half-life. Ceftizoxime sodium is very stablein the dry state. Solutions maintain potency for up to 24hours at room temperature and 10 days when refrigerated.
Properties of Ceftizoxime sodium
| Melting point: | 227℃ |
| RTECS | XI0368000 |
| storage temp. | -20°C |
| solubility | DMSO: soluble1mg/mL |
| form | solid |
| color | white to faint yellow |
| Water Solubility | Soluble in water |
| Stability: | Unstable in Solution |
| CAS DataBase Reference | 68401-82-1(CAS DataBase Reference) |
Safety information for Ceftizoxime sodium
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ceftizoxime sodium
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Ceftizoxime sodium 95% CAS 68401-82-1View Details
Ceftizoxime sodium 95% CAS 68401-82-1View Details
68401-82-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
