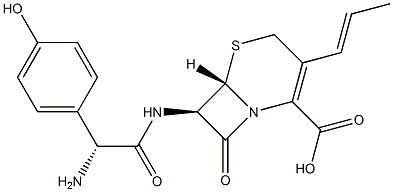
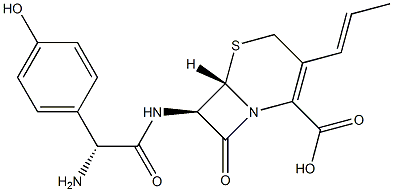
CEFADROXIL
- CAS NO.:50370-12-2
- Empirical Formula: C16H17N3O5S
- Molecular Weight: 363.39
- MDL number: MFCD00865091
- EINECS: 256-555-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
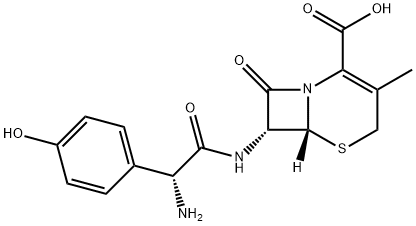
What is CEFADROXIL?
Absorption
Cefadroxil is well absorbed on oral administration; food does not interfere with its absorption.
Toxicity
Nausea, vomiting, diarrhoea, allergic rashes may occur
Description
Cephadroxil is an orally administered antibiotic, which is very similar to cephalexin. After oral administration, its peak serum level is slightly lower than that of cephalexin, but it is excreted more slowly. Therefore, it can be used for oral administration at 8- or 12-hourly intervals (Buck and Price, 1977; Pfeffer et al., 1977). Cephadroxil is still available in a number of European countries (for example, Spain, Belgium, Luxembourg, Portugal, and Finland, among others).
Chemical properties
Cefadroxil is a light orange colored powder. It is a cephalosporin antibiotic and is used for the treatment of bacterial infections. Cefadroxil is stable under recommended storage conditions. Cefadroxil is not considered hazardous when handled under normal medical conditions and with good housekeeping
Originator
Oracefal,Bristol,France,1977
The Uses of CEFADROXIL
Antibacterial;Bacterial transpeptidase inhibitor
Indications
For the treatment of the following infections (skin, UTI, ENT) caused by; S. pneumoniae, H. influenzae, staphylococci, S. pyogenes (group A beta-hemolytic streptococci), E. coli, P. mirabilis, Klebsiella sp, coagulase-negative staphylococci and Streptococcus pyogenes
Background
Long-acting, broad-spectrum, water-soluble, cephalexin derivative.
Definition
ChEBI: A cephalosporin bearing methyl and (2R)-2-amino-2-(4-hydroxyphenyl)acetamido groups at positions 3 and 7, respectively, of the cephem skeleton.
Manufacturing Process
1.8 g of sodium N-(1-methoxycarbonyl-1-propen-2-yl)-D(-)-α-amino-(4-
hydroxyphenyl)acetate was suspended in 10 ml of acetone, and one droplet of
N-methylmorpholine was added thereto, and the mixture was cooled to -15°C.There was added 0.85 g of ethyl chlorocarbonate thereto, and the mixture
was reacted at -13°C to -10°C for 30 minutes, and then the reaction solution
was cooled to -20°C.
On the other hand, 1 g of 7-amino-3-methyl-3-cephem-4-carboxylic acid was
suspended in 20 ml of methanol, and 1.4 g of triethylamine was added
thereto to be dissolved, and 0.4 ml of acetic acid was further added thereto.
This solution was cooled to -20°C and the mixed acid anhydride prepared
previously was added thereto. After the mixture was reacted at -20°C for 1
hour, the temperature of the reaction mixture was raised to 0°C over a period
of 1 hour, and the mixture was reacted for 3 hours at the same temperature.
After the reaction, 1 ml of water was added to the reaction mixture, and the
mixture was adjusted to a pH of 1.0 with concentrated hydrochloric acid while
being cooled, and then stirred for 30 minutes, The insoluble matters were
filtered off, and the filtrate was adjusted to a pH of 5.5 with triethylamine.
This solution was concentrated under reduced pressure, and the residue was
diluted with 20 ml of acetone to precipitate white crystals. The crystals were
collected by filtration and washed with ethanol to obtain 1.46 g of white
crystals of 7-[D(-)-α-amino-(4-hydroxyphenyl)acetamido]-3-methyl-3-
cephem-4-carboxylic acid having a decomposition point of 197°C.
Therapeutic Function
Antibacterial
Health Hazard
Exposures to cefadroxil cause certain common side effects. These include nausea, vomiting, stomach disorders, rashes, itching, unusual tiredness or weakness, yellowing of the skin or eyes, red, swollen, or blistered skin, unusual bruising or bleeding, sore throat, respiratory distress, tightness in the chest, swelling of the mouth, face, lips, or tongue, decreased urination, dark urine, vaginal itching, odor, or discharge, fever, chills, joint pain, and seizures. Prolonged or long-term use of cefadroxil should be avoided.
Pharmacokinetics
Cefadroxil, a first-generation cephalosporin antibiotic, is used to treat urinary tract infections, skin and skin structure infections, pharyngitis, and tonsillitis.
Metabolism
Not Available
Precautions
Cefadroxil should be used only under proper medical health care since it has properties of penicillin allergy, renal function, gastrointestinal tract damage. Pregnant women and breast-feeding women should avoid exposure to cefadroxil.
Properties of CEFADROXIL
| Melting point: | 197°C (rough estimate) |
| Boiling point: | 238°C (rough estimate) |
| Density | 1.59 |
| refractive index | 1.6390 (estimate) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | Water:9.17(Max Conc. mg/mL);25.23(Max Conc. mM) |
| pka | 3.12±0.50(Predicted) |
| form | powder or crystals |
| color | White to off-white |
| CAS DataBase Reference | 50370-12-2 |
Safety information for CEFADROXIL
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CEFADROXIL
| InChIKey | BOEGTKLJZSQCCD-UEKVPHQBSA-N |
CEFADROXIL manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


![7-AMINO-3-[(Z)-PROP-1-ENYL]-3-CEPHEM-4-CARBOXYLIC ACID](https://img.chemicalbook.in/CAS/GIF/106447-44-3.gif)
You may like
-
 50370-12-2 Cefadroxil 99%View Details
50370-12-2 Cefadroxil 99%View Details
50370-12-2 -
 50370-12-2 98%View Details
50370-12-2 98%View Details
50370-12-2 -
 Cefadroxil 98% (HPLC) CAS 50370-12-2View Details
Cefadroxil 98% (HPLC) CAS 50370-12-2View Details
50370-12-2 -
 Cefadroxil 95.00% CAS 50370-12-2View Details
Cefadroxil 95.00% CAS 50370-12-2View Details
50370-12-2 -
 Cefadroxil CAS 50370-12-2View Details
Cefadroxil CAS 50370-12-2View Details
50370-12-2 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
