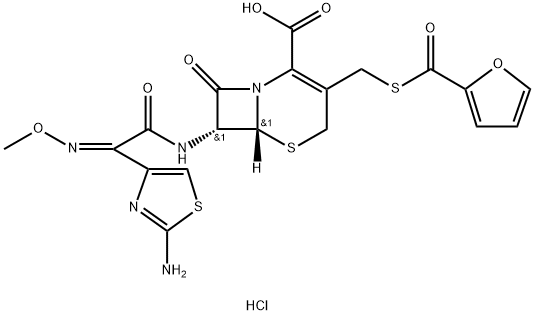
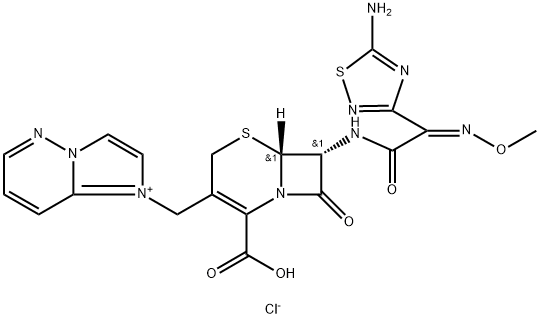
Ceftiofur hydrochloride
Synonym(s):;3-[(2-Furylcarbonyl)thio]methyl]-7-[2-(2-amino-4-thiazolyl)-2-(methoxyiminoacetamido)-3-cephem-4-carboxylic acid hydrochloride;Ceftiofur hydrochloride
- CAS NO.:103980-44-5
- Empirical Formula: C19H18ClN5O7S3
- Molecular Weight: 560.01
- MDL number: MFCD04038020
- EINECS: 600-507-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-18 11:59:04
What is Ceftiofur hydrochloride?
Description
Ceftiofur Hydrochloride is the hydrochloride salt form of ceftiofur, a semisynthetic, beta-lactamase-stable, broad-spectrum, third-generation cephalosporin with antibacterial activity. Ceftiofur binds to and inactivates penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. PBPs are enzymes involved in the terminal stages of assembling the bacterial cell wall and in reshaping the cell wall during growth and division. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This results in the weakening of the bacterial cell wall and causes cell lysis.
Chemical properties
Off-White Solid
The Uses of Ceftiofur hydrochloride
Ceftiofur hydrochloride (ceftiofur) has been approved for treatment against respiratory diseases in many animals.1,3,8–10 The recommended dosage regimen of ceftiofur for the treatment of swine respiratory disease is 3–5 mg/kg body weight (BW) administered intramuscularly once daily for 3–5 consecutive days.
The Uses of Ceftiofur hydrochloride
An antibacterial.
What are the applications of Application
Ceftiofur Hydrochloride is a cephalosporin antibiotic resistant to beta-lactamase
What are the applications of Application
Ceftiofur hydrochloride is used for the treatment and control of infections in cattle and pigs caused by susceptible pathogens. It is approved for the treatment of bovine respiratory disease caused by Bartonella mansoni, Bartonella multocida and Bartonella somniferum (formerly Bartonella somniferum).
Ceftiofur hydrochloride is also used for the treatment of bovine interdigital necrotizing bacillosis (rotten foot disease) caused by Fusobacterium necrophorum or Bacteroides melaninogenicus. Ceftiofur hydrochloride has been shown to be effective at a dose of 2.2 mg/kg for the treatment of acute postpartum onychomycosis in dairy cows or by intrauterine instillation (1 g) for the treatment of retained foetal membranes.
Ceftiofur hydrochloride can be used for the treatment of SRD caused by Actinobacillus, Plasmodium multocida, Salmonella cholera and Streptococcus suis.Ceftiofur hydrochloride and ceftiofur amorphous acid can also be administered via the intra-udder route in dairy cows. For intramammary use, a specific formulation (Spectramast DC) is recommended. Although ceftiofur sodium has been used to treat infections in horses and dogs, there is little experience with the administration of ceftiofur sodium hydrochloride in these species.
Veterinary Drugs and Treatments
In swine, ceftiofur HCl injection is labeled for the treatment and
control of swine bacterial respiratory disease (swine bacterial
pneumonia) associated with Actinobacillus (Haemophilus) pleuropneumoniae,
Pasteurella multocida, Salmonella choleraesuis and
Streptococcus suis.
In cattle, ceftiofur HCl is labeled for the treatment of the following
bacterial diseases: Bovine respiratory disease (BRD, shipping
fever, pneumonia) associated with Mannheimia haemolytica,
Pasteurella multocida, and Histophilus somni; Acute bovine interdigital
necrobacillosis (foot rot, pododermatitis) associated with
Fusobacterium necrophorum and Bacteroides melaninogenicus; and
acute metritis (0 – 14 days post-partum) associated with bacterial
organisms susceptible to ceftiofur.
The intramammary syringe for dry dairy cattle (Spectramast
DC?) is labeled for the treatment of subclinical mastitis in dairy
cattle at the time of dry off associated with Staphylococcus aureus,
Streptococcus dysgalactiae, and Streptococcus uberis. The intramammary
syringe for lactating dairy cattle (Spectramast LC?) is labeled
for the treatment of clinical mastitis in lactating dairy cattle associated
with coagulase-negative staphylococci, Streptococcus dysgalactiae,
and Escherichia coli.
Properties of Ceftiofur hydrochloride
| Melting point: | >190°C (dec.) |
| alpha | -92~-98°(D/20℃)(c=0.2,CH3OH) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | neat |
| color | Off-White |
Safety information for Ceftiofur hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. P362:Take off contaminated clothing and wash before reuse. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P370+P378:In case of fire: Use … for extinction. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P403+P235:Store in a well-ventilated place. Keep cool. P501:Dispose of contents/container to..… |
Computed Descriptors for Ceftiofur hydrochloride
| InChIKey | KEQFDTJEEQKVLM-JUODUXDSSA-N |
| SMILES | C(C1=C(CS[C@]2([H])[C@H](NC(=O)/C(/C3N=C(N)SC=3)=N\OC)C(=O)N12)CSC(C1OC=CC=1)=O)(=O)O.Cl |&1:5,7,r| |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 103980-44-5 Ceftiofur hydrochloride 98%View Details
103980-44-5 Ceftiofur hydrochloride 98%View Details
103980-44-5 -
 103980-44-5 99%View Details
103980-44-5 99%View Details
103980-44-5 -
 CEFTIOFUR HYDROCHLORIDE CAS 103980-44-5View Details
CEFTIOFUR HYDROCHLORIDE CAS 103980-44-5View Details
103980-44-5 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
