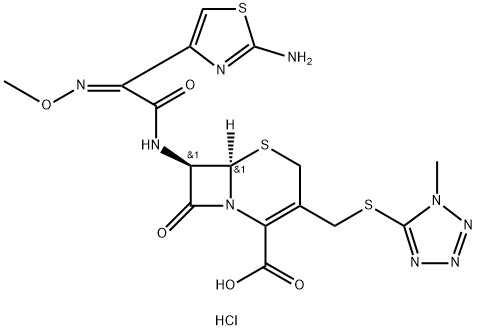
Cefozopran hydrochloride
- CAS NO.:113981-44-5
- Empirical Formula: C19H18ClN9O5S2
- Molecular Weight: 551.98
- MDL number: MFCD00944908
- EINECS: 1806241-263-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
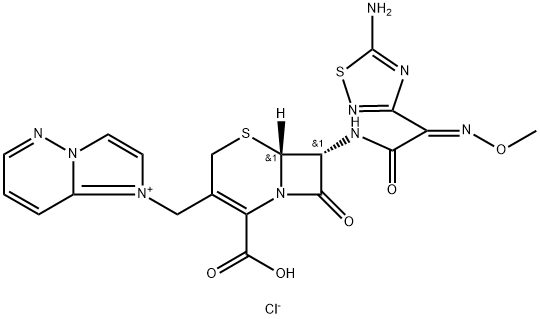
What is Cefozopran hydrochloride?
Antimicrobial activity
Cefazolam hydrochloride is suitable for Staphylococcus, Streptococcus, Enterococcus, Peptostreptococcus, Escherichia coli, Enterobacter, Serratia, Klebsiella, Citrobacter, Proteus, Pseudomonas, Influenza Bacillus, Acinetobacter, Bacteroidetes, septicemia caused by sensitive bacteria to cefazolam hydrochloride, trauma infection, respiratory system, urinary system and abdominal cavity, pelvic purulent inflammation, ophthalmology and ENT inflammation, etc.
Biological Activity
Cefozopran (SCE-2787) hydrochloride is a semi-synthetic, parenteral, fourth-generation cephalosporin. As an antibiotic, it has broad-spectrum antibacterial activity and can inhibit most Gram-negative and Gram-positive bacteria.
in vitro
Cefozopran (SCE-2787) is a fourth-generation cephalosporin that has good activity against gram-positive organisms including methicillin-susceptible staphylococci, enterococci, and viridans group streptococci; and against gram-negative organisms including hemophilus influenza. Moreover, cefozopran has comparatively good activity against enterococci and P. aeruginosa , which are refractory to other cephalosporins.
in vivo
Cefozopran (SCE-2787) (5-80 mg/kg; sc; twice a day for 5 days; four-week-old ICR male mice) is effective against acute respiratory tract infections caused by Kiebsiella pneumonia DT-S. In the model of chronic respiratory tract infection caused by K. pneumoniae 27, Cefozopran (20-80 mg/kg; sc; twice a day for 7 days; five-week-old CBA/J female mice) is as effective as Ceftazidime.
Properties of Cefozopran hydrochloride
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| form | Solid |
| color | White to light yellow |
| Water Solubility | Water : ≥ 52 mg/mL (94.20 mM) |
Safety information for Cefozopran hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cefozopran hydrochloride
Abamectin manufacturer
VIVAN Life Sciences Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 Cefozopran hydrochloride 98%View Details
Cefozopran hydrochloride 98%View Details -
 113981-44-5 99%View Details
113981-44-5 99%View Details
113981-44-5 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4