Cefonicid sodium
- CAS NO.:61270-78-8
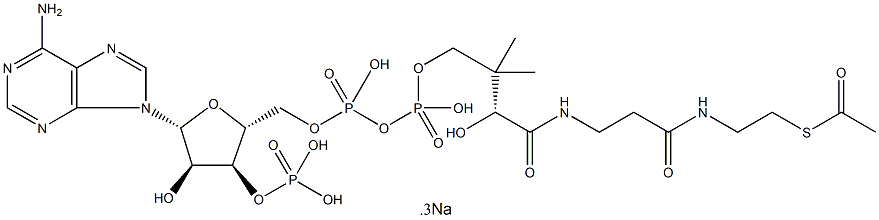
- Empirical Formula: C18H19N6NaO8S3
- Molecular Weight: 566.55
- MDL number: MFCD00941437
- EINECS: 612-109-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
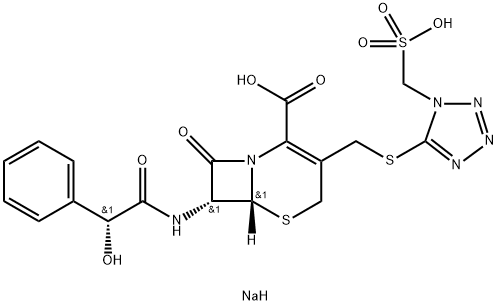
What is Cefonicid sodium?
Description
Cefonicid sodium is the first of the second-generation cephalosporin antibiotics with a long enough serum half-life b4.5 hr.) to allow once-daily i.v. administration. Cefonicid is resistant to β-lactamases produced by S. aureus, H. influenzae, -N. gonorrhea, and related organisms. As with other drugs of this class, it is useful in the treatment of urinary tract and lower respiratory tract infections, skin and skin structure infections, and bone and joint infections, as well as in surgical prophylaxis.
Description
Cefonicid is a second-generation cephalosporin antibiotic. Like other cephalosporins, cefonicid interferes with cell wall biosynthesis in bacteria, leading to lysis of the infectious organism. It is a broad-spectrum antibiotic and has a prolonged serum elimination half-life in animals.
Chemical properties
White Powder
Originator
Smith Kline & French (USA)
The Uses of Cefonicid sodium
A semi-synthetic cephalosporin antibiotic related to Cefamandole. Antibacterial.
Definition
ChEBI: Cefonicid sodium is an organic sodium salt. It contains a cefonicid(2-).
What are the applications of Application
Cefonicid sodium is a broadspectrum cephalosporin antibiotic
brand name
MONOCID
Clinical Use
Cefonicid Sodium (Monocid) is a second-generationcephalosporin that is structurally similar to cefamandole,except that it contains a methane sulfonic acid groupattached to the N-1 position of the tetrazole ring. Theantimicrobial spectrum and limited β-lactamase stabilityof cefonicid are essentially identical with those ofcefamandole.
Cefonicid is unique among the second-generationcephalosporins in that it has an unusually long serum halflifeof approximately 4.5 hours. High plasma protein bindingcoupled with slow renal tubular secretion are apparentlyresponsible for the long duration of action. Despite the high fraction of drug bound in plasma, cefonicid is distributedthroughout body fluids and tissues, with the exception of thecerebrospinal fluid.
Cefonicid is supplied as a highly water-soluble disodiumsalt, in the form of a sterile powder to be reconstituted forinjection. Solutions are stable for 24 hours at 25°C and for72 hours when refrigerated.
in vitro
cefonicid was found to be similar to cefamandole in its superiority to first generation cephalosporins against several enterobacteriaceae and haemophilus influenzae, such as beta-lactamase-producing strains. its activity against staphylococcus aureus was similar to that of cefoxitin and inferior to cefamandole and first generation cephalosporins. cefonicid has excellent in-vitro activity against neisseria gonorrhoeae, but is inactive against pseudomonas, serratia, acinetobacter, and bacteroides fragilis [1].
in vivo
the purpose of a previous study was to determine whether cefonicid caused testicular toxicity when subcutaneously administered to sprague-dawley male rats at doses of 50 to 1,000 mg/kg per day. the histological findings were confirmed by marked reductions in testicular sperm production rates and cauda epididymal sperm numbers. in addition, cefonicid had no treatment-related adverse effects on the sexual maturation males [2].
References
[1] saltiel, e; brogden, r. n. (1986). cefonicid. a review of its antibacterial activity, pharmacological properties and therapeutic use. drugs. 32 (3): 222–59.
[2] manson jm, zolna le, kang yj, johnson cm. effects of cefonicid and other cephalosporin antibiotics on male sexual development in rats. antimicrob agents chemother. 1987 jul;31(7):991-7.
Properties of Cefonicid sodium
| Melting point: | > 160°C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Pale Yellow |
| CAS DataBase Reference | 61270-78-8(CAS DataBase Reference) |
Safety information for Cefonicid sodium
Computed Descriptors for Cefonicid sodium
Cefonicid sodium manufacturer
Aruvi Labs
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 61270-78-8 Cefonicid disodium 98%View Details
61270-78-8 Cefonicid disodium 98%View Details
61270-78-8 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1