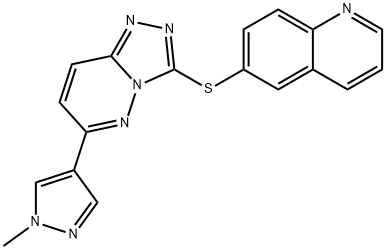
Capmatinib
- CAS NO.:1029712-80-8
- Empirical Formula: C23H17FN6O
- Molecular Weight: 412.42
- MDL number: MFCD18633285
- EINECS: 813-241-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
What is Capmatinib?
Absorption
The oral bioavailability of capmatinib is estimated to be >70%. Following oral administration, maximum plasma concentrations are achieved within 1 to 2 hours (Tmax). Co-administration with a high-fat meal increased capmatinib AUC by 46% with no change in Cmax (as compared to fasted conditions), and co-administration with a low-fat meal had no clinically meaningful effects on exposure.
Toxicity
Data regarding toxicity and overdose of capmatinib are limited. Embryo-fetal toxicity has been documented in animal models - both males and females using capmatinib should use effective contraception throughout the course of therapy and for 1 week following cessation of therapy.
Description
Capmatinib is a competitive inhibitor with very potent and selective activity against MET compared to other kinases.
Description
INCB 28060 is an inhibitor of heptatocyte growth factor receptor (HGFR, also known as c-Met), potently blocking in vitro kinase activity (IC50 = 0.13 nM) as well as constitutive or HGF-stimulated activity in cells (IC50 values range from 0.3 to 1.1 nM). It blocks cell proliferation and migration or induces apoptosis in different types of cancer cells. INCB 28060 is orally bioavailable and inhibits the growth of HGFR-dependent tumors in mice. It also improves efficacy of gemcitabine in a mouse pancreatic cancer model and reduces migration and adhesion in ovarian cancer cell models.
The Uses of Capmatinib
2-Fluoro-N-methyl-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzamide is used in the preparation of a combination formulation with allosteric SHP2 inhibitor TNO155. Used in treatment of non-small cell lung cancer with specific mutations (those that lead to mesenchymal-epithelial transition or MET exon 14 skipping).
Background
Capmatinib is a small molecule kinase inhibitor targeted against c-Met (a.k.a. hepatocyte growth factor receptor [HGFR]), a receptor tyrosine kinase that, in healthy humans, activates signaling cascades involved in organ regeneration and tissue repair. Aberrant c-Met activation - via mutations, amplification, and/or overexpression - is known to occur in many types of cancer, and leads to overactivation of multiple downstream signaling pathways such as STAT3, PI3K/ATK, and RAS/MAPK. Mutations in MET have been detected in non-small cell lung cancer (NSCLC), and the prevalence of MET amplification in epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI)-naive patients with NSCLC has been reported to be 1.4% - 21%. This co-occurrence has made c-Met a desirable target in the treatment of NSCLC.
Manufactured by Novartis and marketed under the brand name Tabrecta, capmatinib was granted accelerated approval by the FDA on May 6, 2020, for the treatment of NSCLC in patients whose tumors have a mutation that leads to mesenchymal-epithelial transition (MET) exon 14 skipping. The presence of the mutation must be confirmed by an FDA-approved test, such as the FoundationOne CDx assay (manufactured by Foundation Medicine, Inc.), which was approved by the FDA on the same day. As this indication was granted under an accelerated approval, its continued approval is contingent upon verification of capmatinib's benefit in confirmatory trials. Capmatinib was approved by Health Canada on June 8, 2022.
Indications
In the US, capmatinib is indicated for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have a mutation that leads to mesenchymal-epithelial transition (MET) exon 14 skipping as detected by an FDA-approved test.
Capmatinib is approved to treat adults with locally advanced unresectable or metastatic non-small cell lung cancer (NSCLC) with MET exon 14 skipping alterations in Canada.
What are the applications of Application
INCB28060 is a novel inhibitor of Met (IC50 = 0.13 nM)
brand name
Tabrecta
General Description
Class: receptor tyrosine kinase; Treatment: NSCLC with METex14; Other name: INCB28060; Oral bioavailability >70%; Elimination half-life = 7.8 h; Protein binding = 96%
Pharmacokinetics
Capmatinib inhibits the overactivity of c-Met, a receptor tyrosine kinase encoded by the MET proto-oncogene. Mutations in MET are involved in the proliferation of many cancers, including non-small cell lung cancer (NSCLC).
Capmatinib may cause photosensitivity reactions in patients following ultraviolet (UV) exposure - patients undergoing therapy with capmatinib should be advised to use sunscreen and protective clothing to limit exposure to UV radiation. Instances of interstitial lung disease/pneumonitis, which can be fatal, occurred in patients being treated with capmatinib. Patients presenting with signs or symptoms of lung disease (e.g. cough, dyspnea, fever) should have capmatinib immediately withheld, and capmatinib should be permanently discontinued if no other feasible causes of the lung-related symptoms are identified.
Side Effects
Most common adverse events were nausea, peripheral edema, decreased appetite, rash, and increased amylase and lipase levels.
in vitro
It has been shown in vitro that cell lines made resistant to erlotinib, an EGFR inhibitor, could be resensitized after capmatinib treatment. Results from a phase Ib/II study of patients with EGFR-mutated, MET-dysregulated NSCLC have shown promising responses to a combination of capmatinib and gefitinib (EGFR TKI) following disease progression from an only EGFR TKI treatment regimen. Recommended phase II dose was determined to be capmatinib 400 mg twice/day and gefitinib 250 mg once/day.
Metabolism
Capmatinib undergoes metabolism primarily via CYP3A4 and aldehyde oxidase. Specific biotransformation pathways and metabolic products have yet to be elucidated.
References
1. liu x, wang q, yang g, et al. a novel kinase inhibitor, incb28060, blocks c-met-dependent signaling, neoplastic activities, and cross-talk with egfr and her-3. clinical cancer research : an official journal of the american association for cancer research. 2011;17(22):7127-7138.
Properties of Capmatinib
| Melting point: | >250°C (dec.) |
| Density | 1.40 |
| vapor pressure | 0-0Pa at 20-25℃ |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 13.82±0.46(Predicted) |
| color | Pale Yellow to Light Yellow |
| InChI | InChI=1S/C23H17FN6O/c1-25-22(31)18-6-5-16(11-19(18)24)21-13-28-23-27-12-17(30(23)29-21)10-14-4-7-20-15(9-14)3-2-8-26-20/h2-9,11-13H,10H2,1H3,(H,25,31) |
Safety information for Capmatinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Capmatinib
| InChIKey | LIOLIMKSCNQPLV-UHFFFAOYSA-N |
| SMILES | C(NC)(=O)C1=CC=C(C2=NN3C(CC4=CC=C5C(=C4)C=CC=N5)=CN=C3N=C2)C=C1F |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4