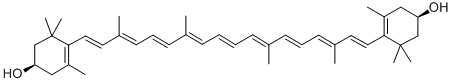
Canthaxanthin
Synonym(s):β-Carotin-4,4′-dione;Canthaxanthin (trans);E 161g
- CAS NO.:514-78-3
- Empirical Formula: C40H52O2
- Molecular Weight: 564.85
- MDL number: MFCD00016364
- EINECS: 208-187-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 08:32:17
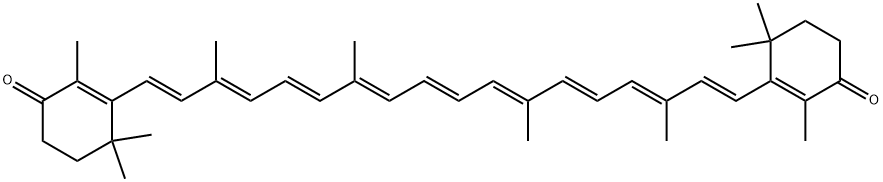
What is Canthaxanthin?
The Uses of Canthaxanthin
Canthaxanthin is a synthetic red colorant that is the carotenoid of most intense red color. it is available in oil-soluble, oil-dispersible, and water-dispersible forms. it has fair ph, heat, light, and chemical stability with a low tinctorial strength. unlike the carotenoids beta- carotene and beta-apo-8-carotenal, it does not possess vitamin a activity. maximum usage level is 66 ppm. uses include carbonated soft drinks, salad dressing, and spaghetti sauce.
The Uses of Canthaxanthin
Antioxidant immune to promote
The Uses of Canthaxanthin
Permissible color additive for food and drugs (exempt from certification): Fed. Regist. 34, no. 5 (Jan. 8, 1969). Oral suntanning agent.
What are the applications of Application
Canthaxanthin is An antioxidant and suppressant of cancer cells
Definition
ChEBI: A carotenone that consists of beta,beta-carotene bearing two oxo substituents at positions 4 and 4'.
brand name
Apotrin;Phenoro.
World Health Organization (WHO)
Canthaxanthin, a naturally-occurring carotenoid with a deep redorange colour, is widely used as a food colouring agent. Since the mid-1970s it has been included in oral 'artificial suntan' preparations. It is also available in preparations used in the treatment of certain photodermatoses. By the mid-1980s its use in such preparations had been associated with the accumulation of crystalline deposits in the retina. Reported functional changes relating to dark adaptation have been of marginal clinical significance and largely reversible. Nevertheless, this has led to the withdrawal of artificial suntan preparations containing canthaxanthin by several regulatory authorities. Preparations for treatment of photodermatoses remain available in some but not all of these countries.
General Description
Canthaxanthin (red diketocarotenoid or 4, 4′-diketo-β-carotene), is a common xanthophyll found naturally in microorganisms and marine organisms, as well as in certain animals. It acts as an antioxidant in living organisms. The potential antioxidant activity of canthaxanthin is due to the presence of conjugated double bonds in its structure. It finds applications in various fields including poultry, fishery, cosmetics, medicine, and pharmaceuticals. In food industries, canthaxanthin can be used as a coloring agent.
Hazard
Oral intake may cause loss of night vision.
Safety Profile
When heated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
Purify canthaxanthin by chromatography on a column of deactivated alumina or magnesium oxide, or on a thin layer plate of silica gel G (M
Properties and Applications
yellow light pink to red yellow light.
Properties of Canthaxanthin
| Melting point: | 217~218℃ |
| Boiling point: | 717.0±40.0 °C(Predicted) |
| Density | 1.003±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly) |
| form | neat |
| form | Solid |
| color | Very Dark Red to Black |
| λmax | λ: 465 nm±5 nm Amax |
| Merck | 13,1758 |
| BRN | 1898520 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 514-78-3(CAS DataBase Reference) |
Safety information for Canthaxanthin
Computed Descriptors for Canthaxanthin
| InChIKey | FDSDTBUPSURDBL-DKLMTRRASA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Canthaxanthin CAS 514-78-3View Details
Canthaxanthin CAS 514-78-3View Details
514-78-3 -
 Canthaxanthin CAS 514-78-3View Details
Canthaxanthin CAS 514-78-3View Details
514-78-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
