Cantharidin
Synonym(s):3a,7a-Dimethylhexahydro-4,7-epoxyisobenzofuran-1,3-dione;7a-Dimethylhexahydro-3a,4,7-epoxyisobenzofuran;Cantharidine;Hexahydro-3a,7a-dimethyl-4,7-epoxyisobenzofuran-1,3-dione
- CAS NO.:56-25-7
- Empirical Formula: C10H12O4
- Molecular Weight: 196.2
- MDL number: MFCD00134968
- EINECS: 200-263-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
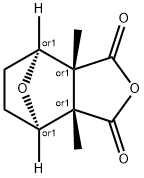
What is Cantharidin?
Absorption
Cantharidin is absorbed from the gastrointestinal tract, and, to a limited extent from the skin as well.
Little pharmacodynamic and pharmacokinetic data regarding cantharidin in the human body currently exists; recruitment for First-Time-In-Human clinical trials regarding such information has been ongoing in 2018. There are however some studies regarding such data in animal models like beagle dogs.
Toxicity
Side effects observed from the topical application of cantharidin include blistering, erythema, pain, bleeding, ring warts, post-inflammatory hyperpigmentation, lymphangitis, secondary bacterial cellulitis, scarring, and varicelliform vesicular dermatitis.
Oral ingestion of cantharidin has resulted in renal failure, blistering and severe damage to the gastrointestinal tract, coagulopathy, seizures, and flaccid paralysis. In the event that YCANTH topical solution is ingested, patients should seek medical attention immediately and contact a Poison Center (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
Carcinogenicity studies have not been conducted with cantharidin. Cantharidin was not mutagenic in a bacteria reverse mutation (Ames) assay. An in vitro chromosomal aberration assay in human lymphocytes with cantharidin was inconclusive. Cantharidin was positive in an in vitro micronucleus assay in TK6 cells, primarily through an aneugenic mechanism. The effects of cantharidin on fertility have not been evaluated.
Description
Cantharidin (56-25-7) inhibits protein phosphatase 2A (Ki=0.19 μM) and PP1 (Ki=1.1 μM).1 Displays >500-fold selectivity over PP2B. Suppresses growth and migration of PANC-1 pancreatic cancer cells via phosphorylation and degradation of β-catenin.2?Cantharidin arrests G2/M transition via JNK/Sp1-dependent down-regulation of CDK1.3?Cell permeable. Warning: Avoid skin contact, may cause skin irritation or blistering.
Chemical properties
white to light yellow crystal powde
Chemical properties
Cantharidin is a brown to black powder.
The Uses of Cantharidin
Cantharidin is a natural toxin produced by blister beetles that moderately inhibits protein phosphatases 1 (PP1) (IC50 = 1.7 μM) and PP2A (IC50 = 0.2 μM) and only weakly inhibits the activity of PP2B (IC50 = 1 mM). It has been shown to stimulate cell cycle progression and induce premature mitosis, used topically (0.7%) as an anti-wart treatment, and has been shown to be active in cervical, tongue, neuroblastoma, bone, leukemia, ovarian, colon, and various other cancer cell lines.
The Uses of Cantharidin
In veterinary medicine as a vesicant, rubefa- cient, and counterirritant.
The Uses of Cantharidin
Cantharidin is used as a potent inhibitor of PP2A phosphatase activity. It has been shown to stimulate cell cycle progression and induce premature mitosis, used topically (0.7%) as an anti-wart treatment, and has been shown to be active in cervical, tongue, neuroblastoma, bone, leukemia, ovarian, colon, and various other cancer cell lines.
Background
Cantharidin is a naturally occurring odorless, colorless fatty substance of the terpenoid class that is produced as an oral fluid in the alimentary canal of the male blister beetle. For its natural purpose, the male blister beetle secretes and presents the cantharidin to a female beetle as a copulatory gift during mating. Post-copulation, the female beetle places the cantharidin over her eggs as protection against any potential predators.
Topical cantharidin products do not necessarily demonstrate any particular better effectiveness at treating topical skin conditions like warts than other commonly available vesicant and/or keratolytics although various studies have also investigated the possibility of using cantharidin as an inflammatory model or in cancer treatment. Regardless, the ongoing lack of FDA approval is likely related to certain toxic effects that were observed following oral ingestion, which include ulceration of the gastrointestinal and genitourinary tracts, along with electrolyte and renal function disturbance in humans and animals .
Available synthetically since the 1950s, topical applications of cantharidin have been used predominantly as a treatment for cutaneous warts since that time. In 1962 however, marketers of cantharidin failed to produce sufficient efficacy data, resulting in the FDA revision of approval of cantharidin. Nevertheless, it gained its first FDA approval on Jul 21, 2023, under the brand name YCANTH? by Verrica Pharmaceuticals for the treatment of molluscum contagiosum in adult and pediatric patients. The approval was based on positive results from 2 phase III trials, CAMP-1 and CAMP-2, where 46% and 52% of patients treated with cantharidin achieved complete molluscum clearance, respectively.
What are the applications of Application
Cantharidin is a toxic herbicidal potent inhibitor of PP2A phosphatase activity
Indications
Cantharidin is approved by the FDA for the topical treatment of molluscum contagiosum in adult and pediatric patients 2 years of age and older. It has also been approved by Health Canada for the treatment of common warts (verruca vulgaris), periungual warts, and molluscum contagiosum as a standalone product and resistant and heavily keratinized plantar warts as a combination product with salicylic acid and podophyllin.
At the same time, such topical cantharidin applications have also been used for a number of off-label indications like callus removal, cutaneous leishmaniasis, herpes zoster, and acquired perforating dermatosis. Furthermore, since most topical cantharidin applications are most commonly available in a 0.7% formulation or a more potent 1% mixture, the 0.7% formulation is most commonly indicated for the treatment of common warts, periungual warts, and molluscum contagiosum while the more potent 1% mixture is typically limited only for use by healthcare professionals in a clinical setting for treating plantar warts and other more specialized off-label conditions.
Moreover, there have also been studies into whether or not cantharidin could be effective at being used as an inflammatory model or in cancer treatment - either of which has yet to elucidate any results formally.
Definition
ChEBI: A monoterpenoid with an epoxy-bridged cyclic dicarboxylic anhydride structure secreted by many species of blister beetle, and most notably by the Spanish fly, Lytta vesicatoria. Natural toxin inhibitor of protein phosphatases 1 and 2A.
Indications
Cantharidin, from the beetle Cantharis vesicatoria, causes intraepidermal vesiculation
and is used in the treatment of warts and other benign cutaneous lesions.
Cantharidin is extremely toxic if taken internally; it should not be prescribed for
at-home use.
Cantharidin (Cantharone), a mitochondrial poison derived from the blister beetle Cantharis vesicatoria, leads to changes in cell membranes, epidermal cell dyshesion, acantholysis, and blister formation and is also useful. Cantharidin can be compounded with salicylic acid and podophyllin resin; occlusion for only 2 hours is needed with this combination. Thick hyperkeratotic lesions should be pared down before painting. The lesion should then be painted with cantharidin, allowed to dry, and covered with Blenderm or other nonporous occlusive tape; 40% salicylic acid plaster may be used to achieve greater activity. The tape is left on for 24 hours or until the area begins to hurt. A blister, often hemorrhagic, will form, break, crust, and fall off in 7 to 14 days; at this time, the lesion is pared down, and any wart remnants are retreated. Because the effect of cantharidin is entirely intraepidermal, no scarring ensues. Ring-like or "donut configuration" recurrences may be seen occasionally after treatment with cantharidin or, at times, following liquid nitrogen therapy. Owing to this agent’s toxicity, application by a physician is recommended. Verrusol, which contains 30% salicylic acid, 5% podophyllin, and 1% cantharidin, may be used in the same manner.
General Description
Brown to black powder or plates or scales. Formerly used as a counterirritant and vesicant. Used for the removal of warts. Used as an experimental anti tumor agent. Active ingredient in spanish fly, a reputed aphrodisiac.
Reactivity Profile
Organic anhydrides, such as Cantharidin, are incompatible with acids, strong oxidizing agents, alcohols, amines, and bases.
Hazard
Extremely toxic; questionable carcinogen; powerful irritant to all cells and tissues; deadly poi- son; respiratory failure; death.
Health Hazard
Cantharidin is classified as super toxic. Probable oral lethal dose in humans is less than 5 mg/kg or a taste of less than 7 drops for a 70 kg (150 lb.) person. It is very toxic by absorption through skin.
Fire Hazard
When heated to decomposition Cantharidin emits acrid smoke and irritating fumes.
Biological Activity
Natural toxin inhibitor of protein phosphatases 1 and 2A (K i values are 1.1 and 0.19 μ M respectively); similar to okadaic acid (9,10-Deepithio-9,10-didehydroacanthifolicin ). Displays > 500-fold selectivity over PP2B.
Biochem/physiol Actions
Inhibitor of protein phosphatase 2A.
Pharmacokinetics
Cantharidin is a natural toxin produced by the blistering beetle that possesses both vesicant (blistering) and keratolytic effects. The substance elicits these effects by inducing acantholysis (loss of intercellular connections) through the targeting of the desmosomal dense plaque, resulting in the detachment of the desmosomes from the tonofilaments. Cantharidin's effectiveness against warts is proposed to be a result of the exfoliation of the wart body as a consequence of the compound's acantholytic action. This acantholytic action generally does not go beyond the epidermal cells so that the basal layer remains intact and minimal effect occurs on the corium. There is consequently no scarring from the topical application of cantharidin.
Potential Exposure
Formerly used as a counterirritant and vesicant. Also used for the removal of benign epithelial growths, e.g., warts. Used as an experimental antitumor agent. The active ingredient in “Spanish fly,” a reputed aphrodisia
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin,remove contaminated clothing and wash immediately withsoap and water. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart actionhas stopped. Transfer promptly to a medical facility. Whenthis chemical has been swallowed, get medical attention. Foringestion, induce vomiting with syrup of ipecac.
Metabolism
Little pharmacodynamic and pharmacokinetic data regarding cantharidin in the human body currently exists; recruitment for First-Time-In-Human clinical trials regarding such information has been ongoing in 2018. There are however some studies regarding such data in animal models like beagle dogs.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area. A regulated, marked area should be established where this chemical is handled, used, or stored incompliance with OSHA Standard 1910.1045.
Shipping
Cantharidin is not specifically listed in the DOT Performance-Oriented Packaging Standards with respect to labeling requirements or restrictions on shipping quantities.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged, and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
References
1) Honkanen (1993) Cantharidin, another natural toxin that inhibits the activity of serine/threonine protein phosphatases types 1 and 2A; FEBS Lett. 330 283 2) Wu et al. (2014) PP2A inhibitors suppress migration and growth of PANC-1 pancreatic cancer cells through inhibition on the Wnt/β-Catenin pathway by phosphorylation and degradation of β-catenin; Oncol. Rep., 32 513 3) Gong et al. (2015) PP2A inhibitors arrest G2/M transition through JNK/Sp1-dependent down-regulation of CDK1 and autophagy up-regulation of p21; Oncotarget, 6 18469
Properties of Cantharidin
| Melting point: | 215-217 °C (lit.) |
| Boiling point: | 253.08°C (rough estimate) |
| Density | 1.0357 (rough estimate) |
| refractive index | 1.4699 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (25 mg/ml warm), acetone (8 mg/ml), ethanol (8 mg/ml warm), chloroform (1:65), ether (1:560), and ethyl acetate (1:150). |
| form | solution |
| color | white |
| Water Solubility | 30mg/L(20 ºC) |
| Merck | 14,1752 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 weeks. |
| CAS DataBase Reference | 56-25-7(CAS DataBase Reference) |
| IARC | 3 (Vol. 10, Sup 7) 1987 |
| NIST Chemistry Reference | Cantharidin(56-25-7) |
| EPA Substance Registry System | Cantharidin (56-25-7) |
Safety information for Cantharidin
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cantharidin
| InChIKey | DHZBEENLJMYSHQ-XCVPVQRUSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Cantharidin 98% (GC) CAS 56-25-7View Details
Cantharidin 98% (GC) CAS 56-25-7View Details
56-25-7 -
 Cantharidin CAS 56-25-7View Details
Cantharidin CAS 56-25-7View Details
56-25-7 -
 Cantharidin CAS 56-25-7View Details
Cantharidin CAS 56-25-7View Details
56-25-7 -
 Cantharidin CAS 56-25-7View Details
Cantharidin CAS 56-25-7View Details
56-25-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1